山奈酚 | 520-18-3
中文名称
山奈酚
中文别名
山柰素;山柰黄酮醇;3,4',5,7-四羟基黄酮水合物;山柰酚-3;四羟基黄酮;百蕊草素Ⅲ;山奈酚水合物;山柰酚
英文名称
kaempferol
英文别名
3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one;kaempherol;3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one;kaemferol;3,4',5,7-tetrahydroxyflavone;3,4′,5,7-tetrahydroxyflavone;3,5,7‑trihydroxy‑2‑(4‑hydroxyphenyl)‑4H‑1‑benzopyran‑4‑one;4′,5,7-trihydroxyflavonol;3,5,7,4'-tetrahydroxyflavone;3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one
CAS
520-18-3
化学式
C15H10O6
mdl
MFCD00016938
分子量
286.241
InChiKey
IYRMWMYZSQPJKC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:276°C
-
沸点:348.61°C (rough estimate)
-
密度:1.2981 (rough estimate)
-
溶解度:乙醇:20 mg/mL
-
LogP:2.685 (est)
-
物理描述:Solid
-
颜色/状态:Yellow needles from alcohol and water
-
蒸汽压力:1.1X10-13mm Hg at 25 °C (est)
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
碰撞截面:162.9 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:21
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:107
-
氢给体数:4
-
氢受体数:6
ADMET
代谢
为了阐明毛地黄素在大肠中的代谢,研究了猪盲肠微生物对其的生物转化。此外,还研究了猪盲肠微生物降解高良姜素(3,5,7-三羟基黄酮)、山奈酚(3,5,7,4'-四羟基黄酮)、芹菜素(5,7,4'-三羟基黄酮)和木犀草素(5,7,3',4'-四羟基黄酮)的效率。通过高效液相色谱(HPLC)-二极管阵列检测、HPLC-电喷雾离子化-串联质谱和高效液相色谱-质谱对形成的代谢物进行了鉴定。盲肠微生物将...山奈酚转化为4-羟基苯乙酸、间苯三酚和4-甲基苯酚;...为了阐明B环上不同羟基化模式对黄酮类化合物降解程度的影响,将高良姜素和山奈酚的转化以及芹菜素和木犀草素的转化分别与槲皮素(3,5,7,3',4'-五羟基黄酮)和黄烷素(5.7-二羟基黄酮)进行了比较。无论黄酮类化合物的亚类如何,B环上4'位置的羟基似乎是对快速分解的必要条件。B环上额外的羟基并不会影响降解程度。
To elucidate the metabolism of hispidulin in the large intestine, its biotransformation by the pig caecal microflora was studied. In addition, the efficiency of the pig caecal microflora to degrade galangin (3,5,7-trihydroxyflavone), kaempferol (3,5,7,4?-tetrahydroxyflavone), apigenin (5,7,4?-trihydroxyflavone), and luteolin (5,7,3?,4?- tetrahydroxyflavone) was investigated. Identification of the formed metabolites was performed by high-performance liquid chromatography (HPLC)-diode array detection, HPLC-electrospray ionization-tandem mass spectrometry, and high-resolution gas chromatography-mass spectrometry. The caecal microflora transformed ... kaempferol to 4-hydroxyphenylacetic acid, phloroglucinol, and 4-methylphenol; ... To elucidate to what extent different hydroxylation patterns on the B-ring influence the degradation degree of flavonoids, the conversions of galangin and kaempferol as well as that of apigenin and luteolin were compared with those of quercetin (3,5,7,3?, 4?-pentahydroxyflavone) and chrysin (5.7-dihydroxyflavone), respectively. Regardless of the flavonoid subclass, the presence of a hydroxy group at the 4?-position seems to be a prerequisite for fast breakdown. An additional hydroxy group at the B-ring did not affect the degradation degree.
来源:Hazardous Substances Data Bank (HSDB)
代谢
大鼠肝细胞对黄酮醇类化合物槲皮素和山奈酚的代谢进行了研究,使用液相色谱与电喷雾质谱联用(LC-ESI MS)技术。槲皮素和山奈酚被广泛代谢(槲皮素98.8 +/- 0.1%,山奈酚81.0 +/- 5.1%,n = 4),在培养后检测到槲皮素的四种葡萄糖苷酸和山奈酚的两种葡萄糖苷酸。与大鼠肝细胞共同培养后形成的槲皮素和山奈酚的葡萄糖苷酸被鉴定为与UDP-葡萄糖苷酸转移酶同型UGT1A9培养后形成的相同物质。此外,分析了服用富含黄酮糖苷的银杏胶囊后的人类志愿者的血浆样本,通过LC-MS检测黄酮糖苷酸和黄酮糖苷的存在。报告了血浆样本中存在黄酮糖苷的证据。结果表明,UGT1A9是代谢黄酮的关键UDP-葡萄糖苷酸转移酶同型,且完整黄酮糖苷的吸收是可能的。
The metabolism of the flavonoids quercetin and kaempferol by rat hepatocytes was investigated using liquid chromatography coupled with electrospray mass spectrometry (LC-ESI MS). Quercetin and kaempferol were extensively metabolized (98.8 +/- 0.1% and 81.0 +/- 5.1% respectively, n = 4), with four glucuronides of quercetin and two of kaempferol being detected after incubation. The glucuronides of quercetin and kaempferol formed upon incubation with rat hepatocytes were identified as the same ones formed after incubation with the UDP-glucuronosyltransferase isoform UGT1A9. In addition, plasma samples from human volunteers taken after consumption of capsules of Ginkgo biloba, a plant rich in flavonoid glycosides, were analysed by LC-MS for the presence of flavonoid glucuronides and flavonoid glycosides. Reported is evidence for the presence of flavonoid glycosides in samples of plasma. The results suggest that UGT1A9 is a key UDP-glucuronosyltransferase isoform for the metabolism of flavonoids, and that absorption of intact flavonoid glycosides is possible.
来源:Hazardous Substances Data Bank (HSDB)
代谢
槲皮素是一种在可食用植物中广泛分布的黄烷醇,它已被证明在缺乏外部代谢系统的条件下对V79细胞具有基因毒性。外部代谢系统的存在,如大鼠肝脏匀浆(S9混合物),会导致其基因毒性增加,这归因于它通过细胞色素P450(CYP)单加氧酶系统转化为更具基因毒性的黄烷醇槲皮素。
Kaempferol is a flavonoid widely distributed in edible plants and has been shown to be genotoxic to V79 cells in the absence of external metabolizing systems. The presence of an external metabolizing system, such as rat liver homogenates (S9 mix), leads to an increase in its genotoxicity, which is attributed to its biotransformation to the more genotoxic flavonoid quercetin, via the cytochrome P450 (CYP) mono-oxygenase system. ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
Kaempferol has known human metabolites that include (2S,3S,4S,5R)-6-[3,5-Dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid and Kaempferol-3-glucuronide.
来源:NORMAN Suspect List Exchange
毒理性
国际癌症研究机构致癌物:山柰酚
IARC Carcinogenic Agent:Kaempferol
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:无法归类其对人类致癌性
IARC Carcinogenic Classes:Group 3: Not classifiable as to its carcinogenicity to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构专著:第31卷:(1983年)某些食品添加剂、饲料添加剂和天然存在物质
IARC Monographs:Volume 31: (1983) Some Food Additives, Feed Additives and Naturally Occurring Substances
来源:International Agency for Research on Cancer (IARC)
毒理性
3, 其对人类致癌性无法分类。
3, not classifiable as to its carcinogenicity to humans. (L135)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
据报道,他莫昔芬是P-糖蛋白(P-gp)和微粒体细胞色素P450(CYP)3A的底物,而山奈酚是P-gp和CYP3A的抑制剂。因此,可以预期山奈酚会影响他莫昔芬的药代动力学。于是,在没有或伴随口服山奈酚(2.5和10 mg/kg)的情况下,口服给予他莫昔芬(10 mg/kg)。在山奈酚的存在下,他莫昔芬从时间零到无穷大的血浆浓度-时间曲线下的总面积(AUC)显著增加,C(max)显著较高,F比没有山奈酚时大得多。口服山奈酚增强了他莫昔芬的口服生物利用度,这可能是由于山奈酚抑制了CYP3A和P-gp。山奈酚的存在并未改变他莫昔芬代谢物4-羟基他莫昔芬的药代动力学参数。这可能是因为在老鼠体内,CYP3A对4-羟基他莫昔芬形成的贡献并不显著。
It has been reported that tamoxifen is a substrate of P-glycoprotein (P-gp) and microsomal cytochrome P450 (CYP) 3A, and kaempferol is an inhibitor of P-gp and CYP3A. Hence, it could be expected that kaempferol would affect the pharmacokinetics of tamoxifen. Thus, tamoxifen was administered orally (10 mg/kg) without or with oral kaempferol (2.5 and 10 mg/kg). In the presence of kaempferol, the total area under the plasma concentration-time curve from time zero to time infinity (AUC) of tamoxifen was significantly greater, C(max) was significantly higher and F was considerably greater than those without kaempferol. The enhanced bioavailability of oral tamoxifen by oral kaempferol could have been due to an inhibition of CYP3A and P-gp by kaempferol. The presence of kaempferol did not alter the pharmacokinetic parameters of a metabolite of tamoxifen, 4-hydroxytamoxifen. This could have been because the contribution of CYP3A to the formation of 4-hydroxytamoxifen is not considerable in rats.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
这项研究旨在评估在健康人类中,通过监测摄入量与排泄量的关系,在食用豆类(Phaseolus vulgaris L.)后,槲皮素的生物利用率。在七名从煮熟的豆类中摄取槲皮素的健康的受试者中,水解黄酮醇的最大排泄量在摄入后2-8小时获得。发现男性和女性受试者之间尿排泄的性别差异分别为槲皮素剂量的6.10+/-5.50%和5.40+/-5.40%。尽管在最高和最低排泄浓度之间发现了6.72倍的人际差异,但所有个体都表现出相似的排泄轮廓。此外,志愿者槲皮素排泄百分比与体重指数之间存在直接相关性,相关指数等于0.80。除两名个体外,所有个体在摄入后2小时都表现出槲皮素排泄的第一个高峰。该研究揭示了在槲皮素摄入后个体间排泄能力的信息,并表明槲皮素可以用作黄酮醇摄入的生物标志物。
The aim of this study was to assess kaempferol bioavailability in healthy humans, after bean (Phaseolus vulgaris L.) consumption through the monitoring of the excretion in relation to intake. In seven healthy subjects receiving kaempferol from cooked bean, maximum excretion of hydrolyzed flavonol was obtained after 2-8 hr. Intersexual variations in urinary excretion were found to be 6.10+/-5.50% and 5.40+/-5.40% of the kaempferol dose for male and female subjects, respectively. Although a 6.72-fold inter-individual variation between the highest and lowest excretion concentrations was found, all individuals exhibited similar excretion profiles. Moreover, a direct correlation between the percentage of kaempferol excreted and the body mass index of volunteers was observed with a correlation index equal to 0.80. All except two individuals exhibited a first peak of kaempferol excretion 2 hr after ingestion. The study reveals information about inter-individual excretion capacity after kaempferol intake and that kaempferol can be used as a biomarker for flavonol consumption.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
...对菊苣中的山奈酚进行药代动力学研究.../在/四名健康男性和四名健康女性中进行了研究。山奈酚从一个相对较低的剂量(9毫克)开始,从菊苣中被吸收,平均最大血浆浓度为0.1微摩尔,在5.8小时时,表明从小肠远端和/或结肠吸收。尽管在最高和最低最大血浆浓度之间观察到了7.5倍个体间差异,但大多数个体的药代动力学特征表现出显著的一致性。这与主要在大肠吸收的其他黄酮类化合物(例如芸香苷)的概况形成对比。在24小时内,平均有1.9%的山奈酚剂量被排泄。大多数受试者还显示了一个早期的吸收峰,可能是对应于在菊苣中以14%水平存在的山奈酚-3-葡萄糖苷。山奈酚-3-葡萄糖醛酸苷是在血浆和尿液中检测到的主要化合物。槲皮素在血浆或尿液中未被检测到,表明山奈酚缺乏I相羟基化。即使在低口服剂量下,山奈酚在人体中的吸收效率也高于槲皮素。血浆中的主要形式是3-葡萄糖醛酸苷,吸收和排泄的个体间差异较低,这表明尿中山奈酚可以作为暴露的生物标志物。
... A pharmacokinetic study of kaempferol from endive ... /was studied in / four healthy males and four healthy females. Kaempferol, from a relatively low dose (9 mg), was absorbed from endive with a mean maximum plasma concentration of 0.1 uM, at a time of 5.8 hr, indicating absorption from the distal section of the small intestine and/or the colon. Although a 7.5-fold interindividual variation between the highest and lowest maximum plasma concentration was observed, most individuals showed remarkably consistent pharmacokinetic profiles. This contrasts with profiles for other flavonoids that are absorbed predominantly from the large intestine (eg rutin). An average of 1.9% of the kaempferol dose was excreted in 24 hr. Most subjects also showed an early absorption peak, probably corresponding to kaempferol-3-glucoside, present at a level of 14% in the endive. Kaempferol-3-glucuronide was the major compound detected in plasma and urine. Quercetin was not detected in plasma or urine indicating a lack of phase I hydroxylation of kaempferol. Kaempferol is absorbed more efficiently than quercetin in humans even at low oral doses. The predominant form in plasma is a 3-glucuronide conjugate, and interindividual variation in absorption and excretion is low, suggesting that urinary kaempferol could be used as a biomarker for exposure.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
十名平均年龄为28岁的成年志愿者一次性口服给予了六片银杏叶提取物。通过反相高效液相色谱法(RP-HPLC)测定了人尿液中不同时期的槲皮素和山奈酚。结果显示,槲皮素的消除速率常数k和吸收速率常数ka略高于山奈酚;槲皮素的吸收半衰期(t(1/2a))、消除半衰期(t(1/2))和达峰时间(t(max))均低于山奈酚,但差异无统计学意义。槲皮素和山奈酚的平均ka值分别为0.61 hr(-1)和0.55 hr(-1),t(1/2a)为1.51 hr和1.56 hr,k为0.37 hr(-1)和0.30 hr(-1),t(1/2)为2.17 hr和2.76 hr,T(max)为2.30 hr和2.68 hr,这意味着槲皮素和山奈酚的吸收和消除分别为0.17%和0.22%。槲皮素和山奈酚主要以葡萄糖苷酸形式从人体尿液中排出。
Ten adult volunteers with an average age 28 years were given a single oral dose of six tablets of Ginkgo biloba extract. Quercetin and kaempferol in different period of human urine were determined by using RP-HPLC. The results showed the elimination rate constant k and the absorption rate constant ka of quercetin were slightly more than that of kaempferol; and the absorption half-life (t(1/2a)), the elimination half-life (t(1/2)) and t(max) of quercetin were less than that of kaempferol, the differences were, however, not statistically significant. The mean values of ka were 0.61 hr(-1) and 0.55 hr(-1), t(1/2a) 1.51 hr and 1.56 hr, k 0.37 hr(-1) and 0.30 hr(-1), t(1/2) 2.17 hr and 2.76 hr, T(max) 2.30 hr and 2.68 hr for quercetin and kaempferol, respectively, which mean absorption and elimination of quercetin and kaempferol are 0.17% and 0.22%, respectively. Quercetin and kaempferol are excreted in the human urine mainly as glucuronides.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
本研究的目标是调查槲皮素和山奈酚是否能够被转运进入原代培养的脑神经元,建立一个实用的HPLC-UV检测方法用于测定神经元中的这两种黄酮醇,并研究它们通过神经元的摄取和传输行为。目前的结果显示,在高浓度10微克/毫升条件下,随着培养时间的延长,神经元中山奈酚的水平呈线性增加然后达到平台期,但在另外两个浓度1微克/毫升和0.1微克/毫升下并未出现此现象。然而,在三个培养浓度下均未检测到神经元中的槲皮素水平,且在细胞中出现了一个新的峰,其保留时间(3.42分钟)比槲皮素(4.65分钟)短。这一现象表明槲皮素可能被转运进入神经元并迅速代谢为某些衍生物。当神经元与含有高剂量山奈酚的培养液一起培养时,山奈酚可以以浓度和时间依赖性的方式被转运进入神经元。培养基中山奈酚的浓度与细胞中山奈酚的浓度之间存在明显相关性,表明随着剂量的增加(10微克/毫升),细胞中山奈酚的摄取也增加。然而,培养基中槲皮素的浓度与细胞中槲皮素的浓度之间存在负相关。结果表明,山奈酚和槲皮素被神经元以不同的方式处理,这可能是它们对原代培养皮层细胞产生不同效果的一个重要因素。
The objective of this study was to investigate whether kaempferol and quercetin could be transported into primary cultured cerebral neurons, to establish a practical HPLC method with UV detection for the two flavonols in the neurons, and to study the uptake and transport behaviors of them through the neurons. The present results showed that the level of kaempferol in the neurons increased linearly and then reached a plateau with incubation time at the high concentration of 10 ?g/mL, but not at the other two concentrations of 1 and 0.1 ug/mL. However, the levels of quercetin in the neurons were not detected at the three incubating concentrations, and there was a new peak detected in the cell whose retention time was shorter (3.42 min) than that of quercetin (4.65 min). This phenomenon suggested that quercetin might be transported into the neurons and then metabolized quickly to some derivative. Kaempferol could be transported into the neurons in a concentration- and time-dependent manner when the neurons were incubated with the culture medium containing kaempferol at the high dose. There was an apparent correlation between the concentrations of kaempferol in the medium and in the cell, indicating that the uptake of kaempferol in the cell increased along with its dose (10 ug/mL). However, there was a negative correlation between the concentrations of quercetin in the medium and in the cell. The results suggested that kaempferol and quercetin were disposed by the neurons at different way, and this might be an important factor for their different effects on primary cultured cortical cells.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3,-
-
海关编码:2914501900
-
危险品运输编号:UN 2811 6.1 / PGIII
-
RTECS号:LK9275200
-
危险标志:GHS06,GHS08
-
危险性描述:H301,H341
-
危险性防范说明:P281,P301 + P310
-
包装等级:III
-
危险类别:6.1
-
储存条件:储存于阴凉、干燥、通风良好的库房内,远离火种和热源,避免阳光直射,并确保包装密封。应将该物品与酸类及食用化学品分开存放,切忌混储。同时,在储区备有合适的材料以收容可能的泄漏物。
SDS
山奈酚水合物 修改号码:6
模块 1. 化学品
产品名称: Kaempferol Hydrate
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 山奈酚水合物
百分比: >97.0%(HPLC)
CAS编码: 520-18-3
俗名: 3,4',5,7-Tetrahydroxyflavone Hydrate
分子式:
C15H10O6·xH2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
山奈酚水合物 修改号码:6
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
光敏, 气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 黄色-深黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 微溶于
山奈酚水合物 修改号码:6
模块 9. 理化特性
[其他溶剂]
溶于: 醚, 乙醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: mmo-sat 166 nmol/plate (-S9)
dni-hmn-fbr 50 mg/L
mNT-hmn-lym 10 mg/L
dna-esc 100 umol/L
致癌性:
IARC = 3 (无法对人类的致癌性进行分类)。
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: LK9275200
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
山奈酚水合物 修改号码:6
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Kaempferol Hydrate
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 山奈酚水合物
百分比: >97.0%(HPLC)
CAS编码: 520-18-3
俗名: 3,4',5,7-Tetrahydroxyflavone Hydrate
分子式:
C15H10O6·xH2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
山奈酚水合物 修改号码:6
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
光敏, 气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 黄色-深黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 微溶于
山奈酚水合物 修改号码:6
模块 9. 理化特性
[其他溶剂]
溶于: 醚, 乙醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: mmo-sat 166 nmol/plate (-S9)
dni-hmn-fbr 50 mg/L
mNT-hmn-lym 10 mg/L
dna-esc 100 umol/L
致癌性:
IARC = 3 (无法对人类的致癌性进行分类)。
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: LK9275200
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
山奈酚水合物 修改号码:6
模块16 - 其他信息
N/A
制备方法与用途
天然多酚——山柰酚(kaempferol,Kae)是一种显著的天然多酚,也是最常见的膳食类黄酮之一。它广泛存在于植物性食物中,如茶、西兰花、飞燕草、金缕梅、葡萄柚、抱子甘蓝、山奈等。
研究表明,山柰酚在多种神经系统疾病中通过减少小胶质细胞激活介导的抗炎和氧化应激作用而发挥神经保护功能。它能清除体内的多余超氧自由基,防止DNA及细胞氧化损伤,展现出抗肿瘤、抗炎、抗氧化和抗衰老等药理活性,并且有助于治疗糖尿病和骨质疏松症。
性状 山柰酚是一种呈棕色至浅黄色结晶粉末;气味微弱,味道辛辣。
作用 山柰酚具有多种营养保健功能,包括抗氧化、抗炎、抗癌、防治糖尿病、动脉粥样硬化及骨质疏松,并能够保护神经、肝脏和心肌。此外,它还能抑制蛋白激酶等。作为保健食品或药品,山柰酚市场前景广阔。
制备
药理作用 山柰黄酮醇具有抗癌、抑制生育、抗癫痫、抗炎、抗氧化剂、解痉、抗溃疡、利胆利尿及止咳等功效。
化学性质 山柰酚是一种黄色结晶粉末,可溶于甲醇、乙醇和DMSO等有机溶剂。来源于山奈根茎、槐角、茶叶、西兰花、葡萄柚等植物材料。
用途 山柰酚是一种有效的破骨细胞骨吸收抑制剂。它能使佛波醇酯处理的小鼠成纤维细胞或v-H-ras-转化的NIH 3T3细胞变形恢复,并显著诱导核DNA退化,伴随脂类过氧化反应及拓扑异构酶I催化的DNA再连接的抑制。
生产方法 植物材料经粉碎后用水或乙醇提取。随后使用聚酰胺进行吸附并洗脱,之后通过层析法与其他黄酮类物质分离或采用高压液相色谱(HPLC)进行纯化。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 高良姜素 galangin 548-83-4 C15H10O5 270.241 鼠李柠檬素 rhamnocitrin 569-92-6 C16H12O6 300.268 5,7,4'-三甲氧基山柰酚 kaempferol 5,7,4'-trimethyl ether 1098-92-6 C18H16O6 328.321 堪非醇 3,4'-二-O-甲醚 ermanin 20869-95-8 C17H14O6 314.295 5,7-二-(苄氧基)-2-(4-(苄氧基)苯基)-3-羟基-4H-苯并吡喃-4-酮 5,7-bis(benzyloxy)-2-(4-(benzyloxy)phenyl)-3-hydroxy-4H-chromen-4-one 23405-70-1 C36H28O6 556.615 5-羟基-4',7-二甲氧基黄酮 5-hydroxy-7,4'-dimethoxyflavone 5128-44-9 C17H14O5 298.295 5,7,4-三甲氧基黄酮 4',5,7-trimethoxyflavone 5631-70-9 C18H16O5 312.322 去甲脱水淫羊藿黄素 8-prenylkaempferol 28610-31-3 C20H18O6 354.359 —— kaempferol 7-O-α-L-rhamnopyranoside 5041-97-4 C21H20O10 432.384 山柰酚-7-O-alpha-L-鼠李糖苷 kaempferol-7-O-α-L-rhamnoside 20196-89-8 C21H20O10 432.384 山奈酚-7-葡萄糖苷 kaempferol 7-glucoside 16290-07-6 C21H20O11 448.383 —— kaempferol 7-O-glucoside 16290-07-6 C21H20O11 448.383 —— 7-O-(E)-p-coumaroylkaempferol-4'-O-α-L-arabinopyranoside 1215845-90-1 C29H24O12 564.502 山柰酚 3-O-alpha-L-阿拉伯呋喃糖苷 kaempferol-3-O-α-arabinofuranoside 5041-67-8 C20H18O10 418.357 阿福豆苷 kaempferol 3-rhamnoside 482-39-3 C21H20O10 432.384 紫云英苷 astragalin 480-10-4 C21H20O11 448.383 三叶豆苷 kaempferol 3-O-galactoside 23627-87-4 C21H20O11 448.383 —— astragalin —— C21H20O11 448.383 —— kaempferol 3-O-β-glucopyranoside-4'-O-β-xylopyranoside —— C26H28O15 580.499 山奈苷 kaempferitrin 482-38-2 C27H30O14 578.527 —— kaempferol 3-O-α-L-arabinopyranoside-7-O-α-L-rhamnopyranoside —— C26H28O14 564.5 —— Kaempferol-3,7-dirhamnosid 482-38-2 C27H30O14 578.527 —— kaempferol 3-O-rhamnoside 7-O-rhamnoside 482-38-2 C27H30O14 578.527 —— kaempferitin —— C27H30O14 578.527 —— nicotiflorin —— C27H30O15 594.526 —— kaempferol-3-O-α-rhamnopyranosyl (1->6)-O-β-glucopyranoside —— C27H30O15 594.526 决明叶苷 kaempferol 3-O-β-D-glucopyranosyl-(1->6)-β-D-glucopyranoside 22149-35-5 C27H30O16 610.526 —— kaempferol 3-O-α-rhamnopyranosyl(1->6)-β-glucopyranosyl(1->6)-β-galactopyranoside —— C33H40O20 756.669 —— kaempferol-3-O-(6''-O-α-L-rhamnopyranosyl)-β-D-glucoside —— C27H30O15 594.526 莰菲醇-3-O-芸香糖苷 kaempferol-3-rutinoside 17650-84-9 C27H30O15 594.526 —— kaempferol 3-O-robinobioside —— C27H30O15 594.526 —— kaempferol 3-O-β-D-galactopyranoside 7-O-α-L-rhamnopyranoside 38784-79-1 C27H30O15 594.526 山柰酚-3,7-二-O-葡萄糖苷 3,7-Di-O-glucosylkaempferol 25615-14-9 C27H30O16 610.526 —— kaempferol 3-O-glucoside 7-O-rhamnoside —— C27H30O15 594.526 —— kaempferol 3-O-β-D-glucopyranosyl-(1→3)-α-L-rhamnopyranoside —— C27H30O15 594.526 —— kaempferol 3-O-β-D-galactopyranosyl-(1→3)-β-D-galactopyranoside 1454577-75-3 C27H30O16 610.526 —— kaempferol 3-O-α-L-rhamnopyranosyl (1->2)-β-D-glucopyranosyl (1->6)-β-D-galactopyranoside —— C33H40O20 756.669 —— kaempherol 3-O-α-L-arabinofuranoside-7-O-α-L-rhamnopyranoside 89946-00-9 C26H28O14 564.5 —— camelliaside A 135095-52-2 C33H40O20 756.669 3-[(2S,3R,4S,5S,6R)-4,5-二羟基-6-(羟基甲基)-3-[(2S,3R,4S,5R,6R)-3,4,5-三羟基-6-(羟基甲基)四氢吡喃-2-基]氧基四氢吡喃-2-基]氧基-5,7-二羟基-2-(4-羟基苯基)苯并吡喃-4-酮 kaempferol 3-O-[β-D-glucopyranosyl-(1->2)-β-D-glucopyranoside] 152390-63-1 C27H30O16 610.526 山茶苷 B camelliaside B 131573-90-5 C32H38O19 726.642 堪非醇 3-新橙皮糖苷 nicotiflorin 32602-81-6 C27H30O15 594.526 —— kaempferol 3-<β-D-glucopyranosyl(1->2)-β-D-glucopyranosyl(1->2)-β-D-glucopyranoside> 80714-53-0 C33H40O21 772.668 —— kaempferol 3-O-β-D-quinovopyranosyl-(1→2)-α-L-rhamnopyranoside. —— C27H30O14 578.527 人参黄酮苷 Panasenoside 31512-06-8 C27H30O16 610.526 山奈酚-3-O-β-D-槐糖苷 kaempferol-3-O-sophoroside 19895-95-5 C27H30O16 610.526 —— clitorin 55804-74-5 C33H40O19 740.669 5,7-二羟基-3-[(2R,3R,4S,5S,6R)-5-羟基-6-(羟基甲基)-3,4-二[[(2S,3R,4R,5R,6S)-3,4,5-三羟基-6-甲基四氢吡喃-2-基]氧基]四氢吡喃-2-基]氧基-2-(4-羟基苯基)苯并吡喃-4-酮 kaempferol 3-O-{αl-L-rhamnopyranosyl(1→6)-[α-L-rhamnopyranosyl-(1→2)]}-β-D-galactopyranoside 109008-28-8 C33H40O19 740.669 槲皮素 3-O-洋槐糖苷 rutin 153-18-4 C27H30O16 610.526 —— kaempferol-3-rutinoside-7-O-α-L-rhamnopyranoside 57526-56-4 C33H40O19 740.669 —— kaempferol 3-O-(6''-O-rhamnosyl)galactoside 7-O-glucopyranoside —— C33H40O20 756.669 刺槐素 robinin 301-19-9 C33H40O19 740.669 —— kaempferol 3-O-(6''-O-rhamnosyl)galactoside 7-O-rhamnoside —— C33H40O19 740.669 黄酮 FLAVONE 525-82-6 C15H10O2 222.243 —— kaempferol 3-O-(2,3,4-tri-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside —— C39H50O25 918.811 —— kaempherol 3-O-[β-D-glucopyranosyl-(1->3)]-α-L-rhamnopyranosyl-7-O-α-L-rhamnopyranoside —— C33H40O19 740.669 山奈酚葡萄糖醛酸苷 kaempferol 3-O-β-D-glucuronide 22688-78-4 C21H18O12 462.367 —— kaemmpferol 3-[β-D-glucopyranosyl-(1<*>6)-β-D-galactopyranoside] 7-[α-L-arabinofuranoside] —— C32H38O20 742.642 —— kaempferol 3-O-β-D-glucopyranosyl-(1'''→4'')-[α-L-rhamnopyranosyl(1''''→6'')]-β-D-glucopyranosyl-7-O-β-D-glucopyranoside 1374330-88-7 C39H50O25 918.811 —— 7-[(α-L-arabinofuranosyl)oxy]-3-{[4-O-(β-D-glucopyranosyl)-β-D-glucopyranosyl]-oxy}-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one kaempferol 3-[β-D-glucopyranosyl-(1<*>4)-β-D-galactopyranoside] 7-[α-L-arabinofuranoside] —— C32H38O20 742.642 —— grosvenorine —— C33H40O19 740.669 —— 7-O-β-D-glucopyranosyl-kaempferol-3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside 78527-48-7 C33H40O20 756.669 —— kaempferol 3-O-β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranoside-7-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside —— C39H50O25 918.811 —— kaempferol 3-O-(2''-O-β-D-glucopyranosyl)-β-D-galactopyranoside-7-O-β-D-glucopyranoside —— C33H40O21 772.668 —— kaempferol 3-(2-O-α-L-arabinopyranosyl-α-L-rhamnopyranoside)-7-O-α-L-rhamnopyranoside 1201171-06-3 C32H38O18 710.643 —— kaempferol 3-O-β-D-glucosyl-(1→2)-β-D-galactoside 7-O-α-L-rhamnoside 1147526-84-8 C33H40O20 756.669 山柰酚-3-O-槐二糖-7-O-葡萄糖苷 kaempferol 3-O-β-D-[β-D-glucopyranosyl-(1→2)-glucopyranoside]-7-O-β-D-glucopyranoside 55136-76-0 C33H40O21 772.668 银锻苷 trans-tiliroside 20316-62-5 C30H26O13 594.529 —— kaempferol-3-O-α-D-xylopyranosyl-(2a→1b)-2a-O-α-D-xylopyranosyl-(2b→1c)-2b-O-α-D-xylopyranosyl-(2c→1d)-2c-O-α-D-xylopyranosyl-2d-hexadecanoate 1449692-21-0 C51H72O23 1053.12 —— kaempferol 3-O-(2,3-di-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside-7-O-α-L-rhamnopyranoside —— C39H50O24 902.812 —— kaempferol 3-glucuronide-7-glucoside —— C27H28O17 624.509 —— kaempferol 3-O-(3'',6''-di-O-E-p-coumaroyl)-β-D-glucopyranoside 74712-68-8 C30H26O13 594.529 —— kaempferol 3-O-(6''-E-p-coumaroyl-β-D-glucopyranoside)-7-O-β-D-glucopyranoside —— C36H36O18 756.671 —— kaempferol 3-O-β-D-apiofuranosyl-(1→2)-α-L-arabinofuranosyl-7-O-α-L-rhamnopyranoside —— C31H36O18 696.616 —— kaempferol-3-O-β-D-[4'''-E-p-coumaroyl-α-L-rhamnosyl(1→ 6)]-galactoside —— C36H36O17 740.672 山柰酚-3-O-(2,6-二-O-反式-对香豆酰基)-beta-D-吡喃葡萄糖苷 kaempferol 3-O-[2'',6''-di-O-(trans-p-coumaroyl)]-β-D-glucopyranoside 121651-61-4 C39H32O15 740.674 羟基木犀草素 8-beta-D-吡喃葡萄糖苷 orientin 27686-36-8 C21H20O12 464.383 —— kaempferol-3-O-β-D-[4'''-E-p-coumaroyl-α-L-rhamnosyl(1 6)]-(4''-E-p-coumaroyl)galactoside —— C45H42O19 886.817 —— kaempferol-3-O-β-D-[4'''-E-p-coumaroyl-α-L-rhamnosyl(1 6)]-(3''-E-p-coumaroyl)-galactoside —— C45H42O19 886.817 —— Kaempferol-3-O-<2Glc-(p-cumaroyl)-α-L-rhamnopyranosyl(1->6)-β-D-glucopyranosid> —— C36H36O17 740.672 —— kaempferol 3-O-[β-D-xylopyranosyl-(1→4)]-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranoside —— C47H54O26 1034.93 —— kaempferol 3-O-[β-D-glucopyranosyl-(1→4)]-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→2)-α-L-rhamnopyranoside —— C57H62O29 1211.1 —— kaempferol 3-O-[β-D-glucopyranosyl(1-3)][β-D-glucopyranosyl(1-3)-α-L-rhamnopyranosyl(1-6)]-(2-O-trans-p-coumaroyl)-β-D-glucopyranoside 1063877-20-2 C48H56O27 1064.96 —— kaempferol 3-O-β-D-glucopyranosyl-7-O-[(6R,2E)-2,6-dimethyl-6-hydroxy-2,7-octadienoyl-(1→ 3)]-α-L-rhamnopyranoside 1448521-59-2 C37H44O17 760.746 —— kaempferol 3-O-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→2)-α-L-rhamnopyranoside-7-O-α-D-glucopyranosyl-(1→6)-β-D-glucopyranoside —— C63H72O34 1373.24 —— kaempferol 3-O-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranoside-7-O-β-D-glucopyranoside —— C48H56O27 1064.96 —— kaempferol 3-O-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranoside-7-O-α-L-rhamnopyranoside —— C48H56O26 1048.96 —— cardamoside A 1401244-18-5 C48H56O27 1064.96 —— kaempferol 3-O-[β-D-glucopyranosyl-(1→4)]-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→2)-α-L-rhamnopyranoside-7-O-β-D-glucopyranoside —— C63H72O34 1373.24 —— kaempferol 3-O-[β-D-xylopyranosyl-(1→4)]-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→2)-α-L-rhamnopyranoside-7-O-α-L-rhamnopyranoside —— C62H70O32 1327.22 —— kaempferol 3-O-[β-D-xylopyranosyl-(1→4)]-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranoside-7-O-α-L-rhamnopyranoside —— C53H64O30 1181.07 —— kaempferol 3-O-[β-D-xylopyranosyl-(1→4)]-[β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→3)]-β-D-6-O-[4-hydroxy-(E)-cinnamoyl]-glucopyranosyl-(1→2)-α-L-rhamnopyranoside-7-O-β-D-glucopyranoside —— C62H70O33 1343.22 —— kaempferol 3-O-β-D-glucopyranosyl-7-O-[(6R,2E)-2,6-dimethyl-6-hydroxy-2,7-octadienoyl-(1→ 2)]-α-L-rhamnopyranoside 1448521-58-1 C37H44O17 760.746 —— kaempferol-3-O-β-D-(2-feruloylglucopyranosyl)(1→6)-[β-D-glucopyranosyl(1→2)]-β-D-glucopyranoside 1399841-85-0 C43H48O24 948.84 —— 7-O-(E)-feruloyl kaempferol-3-(2,6-di-O-acetyl-β-D-glucopyranoside) 1174172-68-9 C35H32O16 708.629 —— kaempferol 3-O-β-neohesperidoside-7-O-[2-O-(trans-feruloyl)]-β-D-glucopyranoside —— C43H48O23 932.84 —— kaempferol 3-O-[(6-O-E-caffeoyl)-β-D-glucopyranosyl-(1→2)]-β-D-galactopyranoside-7-O-β-D-glucuropyranoside —— C42H44O25 948.796 —— kaempferol 3-O-(2''-O-galloyl)-glucuronide 1104525-47-4 C28H22O16 614.473 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 山奈素 4'-O-methylkaempferol 491-54-3 C16H12O6 300.268 鼠李柠檬素 rhamnocitrin 569-92-6 C16H12O6 300.268 3,5-二羟基-4',7-二甲氧基黄酮 4',7,-di-O-methylkaempferol 15486-33-6 C17H14O6 314.295 槲皮素 quercetol 117-39-5 C15H10O7 302.24 —— 7-O-allylkaempherol 1307876-11-4 C18H14O6 326.306 —— 3,7-dihydroxy-5,4'-dimethoxyflavone 33554-54-0 C17H14O6 314.295 —— kaempferol 4'-sulfate 62369-23-7 C15H10O9S 366.305 5,7,4'-三甲氧基山柰酚 kaempferol 5,7,4'-trimethyl ether 1098-92-6 C18H16O6 328.321 4-乙烯基氯苄 3-methoxy-5,7,4'-trihydroxyflavone 1592-70-7 C16H12O6 300.268 六羟黄酮/栎草亭 quercetagetin 90-18-6 C15H10O8 318.24 堪非醇 3,4'-二-O-甲醚 ermanin 20869-95-8 C17H14O6 314.295 —— 4',7-di-O-benzyl-kaempferol 30652-27-8 C29H22O6 466.49 莰非醇-3,7,4’-三甲醚 5-hydroxy-3,4',7-trimethoxyflavone 15486-34-7 C18H16O6 328.321 —— kaempferol-7-O-sulfate 56857-59-1 C15H10O9S 366.305 —— 4'-O-benzyl-7-O-allylkaempferol 1307876-15-8 C25H20O6 416.43 四甲基山奈酚 3,4',5,7-tetramethoxyflavone 16692-52-7 C19H18O6 342.348 —— 5,4'-di-O-acetyl-kaempferol 1374991-22-6 C19H14O8 370.315 山柰酚三-O-甲氧基甲基醚 3,4',7-tris-O-methoxymethylkaempferol 143724-66-7 C21H22O9 418.4 —— Apigenin acetate —— C17H12O7 328.27 —— 3-O-geranylkaempferol 1246764-73-7 C25H26O6 422.478 甘草黄酮醇 Licoflavonol 60197-60-6 C20H18O6 354.359 —— 7-O-labda-8(17),13E-dienylkaempferol 1246764-78-2 C35H42O6 558.715 —— 3,7,4'-tri-O-methyl-5-O-acetyl-kaempferol 107480-00-2 C20H18O7 370.359 —— 3,7-O-digeranylkaempferol 1246764-71-5 C35H42O6 558.715 —— 5,7-dihydroxy-3,3',4',6-tetramethoxyflavone 35688-42-7 C19H18O8 374.347 —— 3,7-di-O-benzyl-kaempferol 1254191-99-5 C29H22O6 466.49 —— 3,7,4'-tri-O-benzylkaempferol —— C36H28O6 556.615 3,4',7-三乙酸堪非醇酯 kaempferol triacetate 143724-69-0 C21H16O9 412.353 —— 3-O-[(E)-(2-oxo-4-phenylbut-3-en-1-yl)]kaempferol 1338577-19-7 C25H18O7 430.414 —— 3,7,4'-tri-O-tert-butyldimethylsilylkaempferol 1246814-11-8 C33H52O6Si3 629.029 —— 3-O-[(E)-(2-oxo-4-(p-tolyl) but-3-en-1-yl)]kaempferol 1338577-20-0 C26H20O7 444.441 —— 3-O-[(E)-4-(4-hydroxyphenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-12-0 C25H18O8 446.413 —— Kaempferol 3,7,4'-tri-O-sulfate —— C15H10O15S3 526.4 5-O-(3-甲基-2-丁烯基)山柰酚三-O-甲氧基甲基醚 5-O-prenyl-3,4',7-tris-O-methoxymethylkaempferol 143724-70-3 C26H30O9 486.519 —— 3-O-labda-8(17),13E-dienylkaempferol 1246764-79-3 C35H42O6 558.715 —— 3-O-[(E)-4-(4-methoxyphenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-14-2 C26H20O8 460.44 —— 3-O-[(E)-4-(4-ethoxyphenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-15-3 C27H22O8 474.467 —— 3-O-[(E)-4-(4-fluorophenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-25-5 C25H17FO7 448.404 —— 3-O-[(E)-4-(4-cyanophenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-23-3 C26H17NO7 455.423 —— 3-O-[(E)-4-(4-butoxyphenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-16-4 C29H26O8 502.521 —— 3,4',5-tri-O-acetylkaempferol —— C21H16O9 412.353 —— 7-O-methyl-3,4',5-tri-O-acetylkaempferol 13323-01-8 C22H18O9 426.38 四乙酸堪非醇酯 3,5,7-triacetoxy-2-(4-acetoxy-phenyl)-chromen-4-one 16274-11-6 C23H18O10 454.39 —— 3-O-[(E)-4-(4-(hexyloxy)phenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-17-5 C31H30O8 530.574 —— 3-O-[(E)-4-(4-isopropylphenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-21-1 C28H24O7 472.494 —— 3-O-[(E)-4-(4-(octyloxy)phenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-18-6 C33H34O8 558.628 —— 3-O-[(E)-2-oxo-4-(4-(trifluoromethyl)phenyl)but-3-en-1-yl]kaempferol 1338577-24-4 C26H17F3O7 498.412 —— 3,7-O-di(labda-8(17),13E-dienyl)kaempferol 1246764-77-1 C55H74O6 831.189 —— 3,4',5-tri-O-acetyl-7-O-allylkaempferol 1307876-12-5 C24H20O9 452.417 —— 3-O-[(E)-4-(4-(tert-butyl)phenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-22-2 C29H26O7 486.521 —— 3-O-[(E)-4-(4-(methylthio)phenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-28-8 C26H20O7S 476.507 —— 3-O-[(E)-4-(4-(benzyloxy)phenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-13-1 C32H24O8 536.538 去甲脱水淫羊藿黄素 8-prenylkaempferol 28610-31-3 C20H18O6 354.359 —— 3-O-[(E)-4-(3,4-dichlorophenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-33-5 C25H16Cl2O7 499.304 山奈酚-4’’-葡萄糖苷 kaempferol-4'-O-glucoside 52222-74-9 C21H20O11 448.383 —— 3-O-[(E)-4-(3-methoxy-4-hydroxyphenyl-)-2-oxobut-3-en-1-yl]kaempferol 1338577-30-2 C26H20O9 476.439 去水淫羊藿黄素 icaritin 118525-40-9 C21H20O6 368.386 淫羊藿素 8-(3"-hydroxy-3"-methylbutyl)-5,7,4'-trihydroxyflavonol 5240-95-9 C20H20O7 372.375 —— 3-O-[(E)-4-(3-bromo-4-methoxyphenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-34-6 C26H19BrO8 539.336 —— 8-C-geranylkaempferol —— C25H26O6 422.478 —— kaempferol 7-α-O-glucoside 16290-07-6 C21H20O11 448.383 山奈酚-7-葡萄糖苷 kaempferol 7-glucoside 16290-07-6 C21H20O11 448.383 —— kaempferol 7-O-glucoside 16290-07-6 C21H20O11 448.383 —— 3-aminoapigenin 681016-83-1 C15H11NO5 285.256 —— 7,4'-di-O-β-D-glucosylkaempferol —— C27H30O16 610.526 —— kaempferol 7-O-β-D-(4-O-methyl) glucoside 1092795-48-6 C22H22O11 462.41 —— 4H-1-benzopyran-4-one, 5-hydroxy-3,7-bis(methoxymethoxy)-2-[4-(methoxymethoxy)phenyl]-6-(3-methyl-2-butenyl)- 143724-72-5 C26H30O9 486.519 —— 3-O-[(E)-4-(3-methoxy-4-benzyloxy phenyl)-2-oxobut-3-en-1-yl]kaempferol 1338577-31-3 C33H26O9 566.564 —— 6,8-diprenyl-kaempferol 54013-24-0 C25H26O6 422.478 —— 8-C-labda-8(17),13E-dienylkaempferol 1046759-46-9 C35H42O6 558.715 —— 7,4'-bis-O-methoxymethyl-8-prenylkaempferol 852247-44-0 C24H26O8 442.466 —— kaempferol 3-O-xyloside —— C20H18O10 418.357 —— kaempferol 3-O-arabinoside —— C20H18O10 418.357 —— 6",6"-dimethyldihydropyran (2",3":7,8)-5,4'-dihydroxyflavonol 28610-34-6 C20H18O6 354.359 —— kaempferol-7-O-β-D-glucuronide 249938-52-1 C21H18O12 462.367 —— kaempferol-7-glucuronide —— C21H18O12 462.367 阿福豆苷 kaempferol 3-rhamnoside 482-39-3 C21H20O10 432.384 三叶豆苷 kaempferol 3-O-galactoside 23627-87-4 C21H20O11 448.383 —— 3-O-8-C-digeranylkaempferol 1246764-72-6 C35H42O6 558.715 紫云英苷 astragalin 480-10-4 C21H20O11 448.383 —— kaempferol-3-O-β-D-galactopyranoside 107163-34-8 C21H20O11 448.383 —— astragalin —— C21H20O11 448.383 去甲基淫羊藿素三-O-甲氧基甲基醚 5-hydroxy-8-prenyl-3,4',7-tris-O-methoxymethylkaempferol 143724-76-9 C26H30O9 486.519 山奈苷 kaempferitrin 482-38-2 C27H30O14 578.527 —— herbacetin 8-O-β-D-glucopyranoside 11021-22-0 C21H20O12 464.383 山柰酚-3,7-二-O-葡萄糖苷 3,7-Di-O-glucosylkaempferol 25615-14-9 C27H30O16 610.526 —— kaempferol 3,7,4'-tri-O-β-glucoside —— C33H40O21 772.668 —— kaempferol 3-O-glucuronide 22688-78-4 C21H18O12 462.367 山奈酚葡萄糖醛酸苷 kaempferol 3-O-β-D-glucuronide 22688-78-4 C21H18O12 462.367 —— (3''S*,5''S*)-8-(3''-methyl-2''-oxopyrrolidin-5''-yl)kaempferol —— C20H17NO7 383.357 —— [(2R,3R,4S,5R,6S)-3,4,5-triacetyloxy-6-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxochromen-3-yl]oxyoxan-2-yl]methyl acetate 877621-21-1 C29H28O15 616.532 —— 3,5,4'-trihydroxy-6'',6''-dimethylpyrano(2'',3'':7,6)-8-(3''',3'''-dimethylallyl)flavone —— C25H24O6 420.462 —— cnidimonin A —— C30H24O9 528.515 淫羊藿次苷I icariside I 56725-99-6 C27H30O11 530.529 —— kaempferol 8-C-glucoside 562068-65-9 C21H20O11 448.383 淫羊藿苷 icariin 489-32-7 C33H40O15 676.672 依普黄酮 Ipriflavone 35212-22-7 C18H16O3 280.323 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:氘代黄酮类化合物及其制备方法摘要:本发明涉及一种氘代黄酮类化合物及其制备方法,包括以下步骤:将黄酮类化合物、碱和重水混合,在惰性气体环境中于90℃以上条件下反应得到氘代黄酮类化合物钠盐,然后加入有机酸酸化,得到氘代黄酮类化合物;其中,黄酮类化合物为黄芩素、黄芩苷、木犀草素、芹菜素、槲皮素、山奈酚、异鼠李素、橙皮素、葛根素和大豆苷元中的至少一种,碱为氘氧化钠和/或氢氧化钠。本发明提供的上述合成方法简单高效,该合成方法在90℃以上条件下即可反应,条件较为温和,同时产率较高,至少可达40%,操作过程简单,催化剂易于获得,生产成本低,可用于大规模的工业化生产。公开号:CN109879847B
-
作为产物:参考文献:名称:Novel Flavonol Glycosides from the Aerial Parts of Lentil (Lens culinaris)摘要:尽管科学文献中详细描述了小扁豆(Lens culinaris)种子的植物化学成分,但关于小扁豆叶和茎中次生代谢物的数据非常有限。我们的研究表明,小扁豆的地上部分是黄酮类化合物的丰富来源。我们分离得到了六种山柰酚和十二种槲皮素苷类,通过核磁共振波谱学和化学方法阐明了它们的结构。这一组包括16种化合物,这些化合物先前未在科学文献中被描述过:槲皮素3-O-β-D-吡喃葡萄糖基(1→2)-β-D-吡喃半乳糖苷-7-O-β-D-吡喃葡萄糖苷(1),山柰酚3-O-β-D-吡喃葡萄糖基(1→2)-β-D-吡喃半乳糖苷-7-O-β-D-吡喃葡萄糖苷(3),以及它们的衍生物4-10,12-15,17,18,这些衍生物被咖啡酸、对香豆酸、阿魏酸或3,4,5-三羟基肉桂酸酯化,以及山柰酚3-O-{[(6-O-E-对香豆酰基)-β-D-吡喃葡萄糖基(1→2)]-α-L-吡喃鼠李糖基(1→6)}-β-D-吡喃半乳糖苷-7-O-α-L-吡喃鼠李糖苷(11)。我们还评估了它们的DPPH自由基清除活性。这可能是关于小扁豆地上部分黄酮类化合物的首次详细描述。DOI:10.3390/molecules191118152
-
作为试剂:描述:参考文献:名称:烟用单体香料Mal-Ile、制备方法及其应用摘要:本发明涉及一种新型烟用单体香料Mal‑Ile、制备方法及其应用。所述Mal‑Ile结构式如下:。本发明对该单体香料进行卷烟物理保润性能测试和成品卷烟内在感官质量评价,发现其不仅可明显提高烟丝的物理保润性能,同时具有显著改善卷烟感官质量的作用,可降低卷烟的刺激性和杂气,提升卷烟烟气圆润感。公开号:CN104341463B
文献信息
-
Primary aminomethyl derivatives of kaempferol: hydrogen bond-assisted synthesis, anticancer activity and spectral properties作者:Shuanglian Cai、Yangyang Kong、Dan Xiao、Yun Chen、Qiuan WangDOI:10.1039/c7ob02927f日期:——products of kaempferol. The formation of appropriate hydrogen bonds between strong nucleophilic amino acids and phenol is essential for the smooth reaction of the SN2 nucleophilic substitution. The SN2 mechanism hypothesis involving a hydrogen bond-assisted process was also supported by the density functional theory (DFT) analysis. An antiproliferative test of synthetic compounds shows the moderate to potent通过涉及曼尼希反应和S N 2亲核取代两个步骤的组合策略合成了山emp酚的一系列伯氨甲基伯衍生物。产物的结构表明,优先的氨基甲基化在山emp酚的A环的C-6或C-8位置,尤其是后者。有趣的是,实验数据表明分子间氢键在山ka酚的主要氨基甲基产物的形成中起关键作用。在强亲核氨基酸和苯酚之间形成适当的氢键对于S N 2亲核取代的平稳反应是必不可少的。在S Ñ密度泛函理论(DFT)分析也支持涉及氢键辅助过程的2种机理假说。合成化合物的抗增殖测试显示,通过CCK-8分析,它对三种人类癌细胞系(HeLa,HCC1954和SK-OV-3)具有中等至有效的细胞毒活性。化合物4e对HeLa细胞显示出选择性的抗增殖活性,且IC50值较低(4.27μm),值得进一步开发。另一个有趣的结果是,大多数金属络合物的最大发射带位于约480 nm处,而Tm和Yb络合物的最大发射带出现在约533 nm处。
-
Accurate Prediction of Glucuronidation of Structurally Diverse Phenolics by Human UGT1A9 Using Combined Experimental and In Silico Approaches作者:Baojian Wu、Xiaoqiang Wang、Shuxing Zhang、Ming HuDOI:10.1007/s11095-012-0666-z日期:2012.6Catalytic selectivity of human UGT1A9, an important membrane-bound enzyme catalyzing glucuronidation of xenobiotics, was determined experimentally using 145 phenolics and analyzed by 3D-QSAR methods. Catalytic efficiency of UGT1A9 was determined by kinetic profiling. Quantitative structure activity relationships were analyzed using CoMFA and CoMSIA techniques. Molecular alignment of substrate structures was made by superimposing the glucuronidation site and its adjacent aromatic ring to achieve maximal steric overlap. For a substrate with multiple active glucuronidation sites, each site was considered a separate substrate. 3D-QSAR analyses produced statistically reliable models with good predictive power (CoMFA: q2 = 0.548, r2 = 0.949, r pred 2 = 0.775; CoMSIA: q2 = 0.579, r2 = 0.876, r pred 2 = 0.700). Contour coefficient maps were applied to elucidate structural features among substrates that are responsible for selectivity differences. Contour coefficient maps were overlaid in the catalytic pocket of a homology model of UGT1A9, enabling identification of the UGT1A9 catalytic pocket with a high degree of confidence. CoMFA/CoMSIA models can predict substrate selectivity and in vitro clearance of UGT1A9. Our findings also provide a possible molecular basis for understanding UGT1A9 functions and substrate selectivity.通过实验使用145种酚类化合物,并通过3D-QSAR方法分析,确定了人UGT1A9的催化选择性。UGT1A9是一种重要的膜结合酶,催化外源性物质的葡糖醛酸化反应。通过动力学分析确定了UGT1A9的催化效率。使用CoMFA和CoMSIA技术分析了定量结构活性关系。通过将葡糖醛酸化位点及其相邻的芳香环重叠,实现了底物结构的最大立体重叠。对于具有多个活性葡糖醛酸化位点的底物,每个位点被视为单独的底物。3D-QSAR分析产生了统计上可靠的模型,具有良好的预测能力(CoMFA:q2=0.548,r2=0.949,r pred 2=0.775;CoMSIA:q2=0.579,r2=0.876,r pred 2=0.700)。通过轮廓系数图阐明了底物中负责选择性差异的结构特征。将轮廓系数图叠加在UGT1A9的同源模型的催化口袋中,能够高度自信地识别UGT1A9的催化口袋。CoMFA/CoMSIA模型可以预测底物的选择性和UGT1A9的体外清除率。我们的发现还提供了理解UGT1A9功能和底物选择性的可能分子基础。
-
[EN] ALKYNE-, AZIDE- AND TRIAZOLE-CONTAINING FLAVONOIDS AS MODULATORS FOR MULTIDRUG RESISTANCE IN CANCERS<br/>[FR] FLAVONOÏDES CONTENANT DE L'ALCYNE, DE L'AZIDE ET DU TRIAZOLE UTILISÉS COMME MODULATEURS DE RÉSISTANCE MULTIPLE AUX MÉDICAMENTS DANS LES CANCERS申请人:UNIV HONG KONG POLYTECHNIC公开号:WO2013127361A1公开(公告)日:2013-09-06A triazole bridged flavonoid dimer compound library was efficiently constructed via the cycloaddition reaction of a series of flavonoid-containing azides (Az 1-15) and alkynes (Ac 1-17). These triazole bridged flavonoid dimers and their precursor alkyne- and azide-continaing flavonoids were screened for their ability to modulate multidrug resistance (MDR) in P-gp-overexpressed cell line (LCC6MDR), MRPl-overexpressed cell line (2008/MRPl) and BCRP-overexpressed cell line (HEK293/R2 and MCF7-MX100). Generally, they displayed very promising MDR reversal activity against P-gp-, MRPl- and BCRP-mediated drug resistance. Moreover, they showed different levels of selectivity for various transporters. Overall, they can be divided into mono-selective, dual-selective and multi-selective modulators for the P-gp, MRPl and BCRP transporters. The EC50 values for reversing paclitaxel resistance (141 - 340 nM) of LCC6MDR cells, DOX (78 - 590 nM) and vincristine (82 - 550 nM) resistance of 2008/MRPl cells and topotecan resistance (0.9 - 135 nM) of HEK293/R2 and MCF7-MX100 cells were at nanomolar range. Importantly, a number of compounds displayed EC50 at or below 10 nM in BCRP-overexpressed cell lines, indicating that these bivalent triazoles more selectively inhibit BCRP transporter than the P-gp and MRPl transporters. Most of the dimers are notably safe MDR chemosensitizers as indicated by their high therapeutic index values.通过对一系列含有三唑基的黄酮类化合物(Az 1-15)和炔烃(Ac 1-17)进行环加成反应,高效构建了一个三唑桥联的黄酮二聚体化合物库。对这些三唑桥联的黄酮二聚体及其前体炔烃和三唑基的黄酮类化合物进行了筛选,以评估它们对P-gp过表达细胞系(LCC6MDR)、MRP1过表达细胞系(2008/MRP1)和BCRP过表达细胞系(HEK293/R2和MCF7-MX100)调节多药耐药性(MDR)的能力。总体而言,它们显示出对P-gp、MRP1和BCRP介导的药物耐药性具有非常有前景的MDR逆转活性。此外,它们对各种转运蛋白显示出不同程度的选择性。总体而言,它们可以分为对P-gp、MRP1和BCRP转运蛋白具有单选择性、双选择性和多选择性调节剂。逆转LCC6MDR细胞对紫杉醇耐药性(141-340 nM)、2008/MRP1细胞对阿霉素(78-590 nM)和长春碱(82-550 nM)耐药性以及HEK293/R2和MCF7-MX100细胞对托泊替康耐药性(0.9-135 nM)的EC50值在纳摩尔范围内。重要的是,许多化合物在BCRP过表达的细胞系中显示出EC50在或低于10 nM,表明这些双价三唑更具选择性地抑制BCRP转运蛋白而不是P-gp和MRP1转运蛋白。大多数二聚体根据其高治疗指数值显示出明显安全的MDR化疗敏感化剂特性。
-
Process for synthesising anthocyanidins申请人:IDB HOLDING S.p.A.公开号:EP0490615A1公开(公告)日:1992-06-17A process is provided for producing anthocyanidins of formula III wherein m represents an integer from 1 to 3, and X⁻ represents an anion, which involves reducing a protected flavonol of formula where each R⁴ represents a C₁#SB-#₁₀ hydrocarbyl group and m is 1 to 3 and converting OR⁴ groups to hydroxy groups.
-
一种山奈酚的制备方法
表征谱图
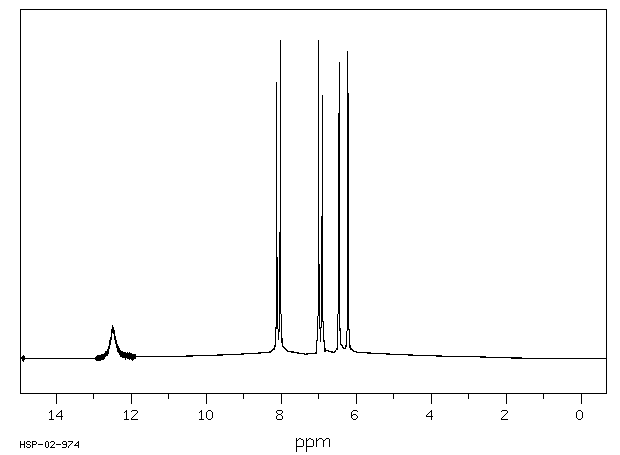
-
氢谱1HNMR
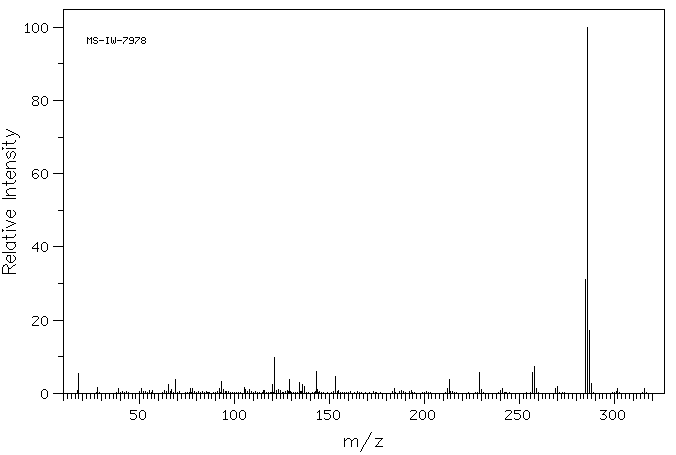
-
质谱MS
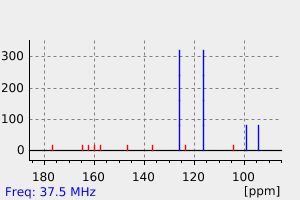
-
碳谱13CNMR
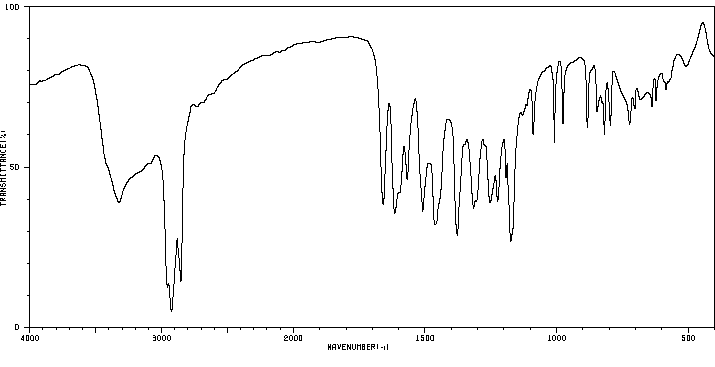
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷