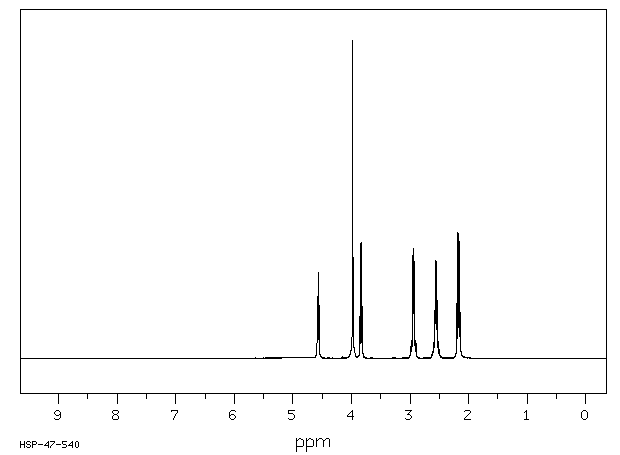
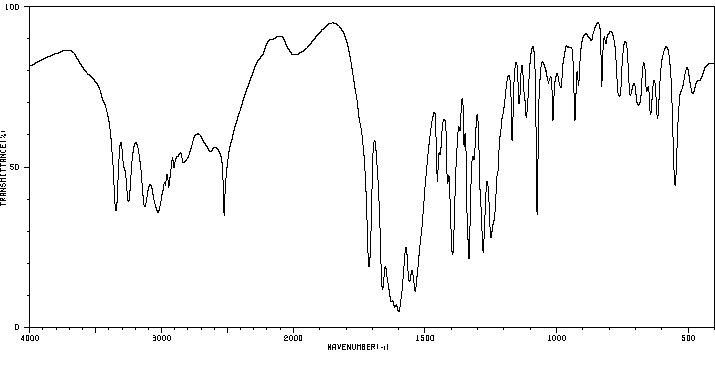
毒理性
谷胱甘肽(GSH)参与白三烯的合成,并且是谷胱甘肽过氧化物酶的辅因子。它还作为一种亲水性分子,在肝脏的生物转化过程中被添加到亲脂性毒素和废物中,之后它们才能成为胆汁的一部分。谷胱甘肽也用于解毒甲基乙二醛,这是一种代谢副产品产生的毒素。这种解毒反应由糖氧酸酶系统完成。糖氧酸酶I催化甲基乙二醛和还原型谷胱甘肽转化为S-D-乳酰谷胱甘肽。糖氧酸酶II催化S-D-乳酰谷胱甘肽转化为还原型谷胱甘肽和D-乳酸。GSH在由细胞质、微体和线粒体中的谷胱甘肽S-转移酶酶催化的结合反应和还原反应中作为辅因子而闻名。然而,它能够参与与非酶结合的一些化学物质,据推测它与N-乙酰-p-苯醌亚胺(NAPQI)有相当程度的结合,NAPQI是由对乙酰氨基酚过量中毒形成的活性细胞色素P450的活性代谢物。在这种能力下,谷胱甘肽作为自杀底物与NAPQI结合,并在过程中解毒它,取代了否则会被有毒加合的细胞蛋白巯基团。对于这种过量的首选医疗治疗方法,在文献中一直得到支持的是雾化形式的N-乙酰半胱氨酸的给药,细胞用它来替换消耗的GSSG并允许一个可用的GSH池。
Glutathione (GSH) participates in leukotriene synthesis and is a cofactor for the enzyme glutathione peroxidase. It is also important as a hydrophilic molecule that is added to lipophilic toxins and waste in the liver during biotransformation before they can become part of the bile. Glutathione is also needed for the detoxification of methylglyoxal, a toxin produced as a by-product of metabolism. This detoxification reaction is carried out by the glyoxalase system. Glyoxalase I catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-Lactoyl-glutathione. Glyoxalase II catalyzes the conversion of S-D-Lactoyl Glutathione to Reduced Glutathione and D-lactate. GSH is known as a cofactor in both conjugation reactions and reduction reactions, catalyzed by glutathione S-transferase enzymes in cytosol, microsomes, and mitochondria. However, it is capable of participating in non-enzymatic conjugation with some chemicals, as it is hypothesized to do to a significant extent with n-acetyl-p-benzoquinone imine (NAPQI), the reactive cytochrome P450 reactive metabolite formed by toxic overdose of acetaminophen. Glutathione in this capacity binds to NAPQI as a suicide substrate and in the process detoxifies it, taking the place of cellular protein sulfhydryl groups which would otherwise be toxically adducted. The preferred medical treatment to an overdose of this nature, whose efficacy has been consistently supported in literature, is the administration (usually in atomized form) of N-acetylcysteine, which is used by cells to replace spent GSSG and allow a usable GSH pool.
来源:Toxin and Toxin Target Database (T3DB)