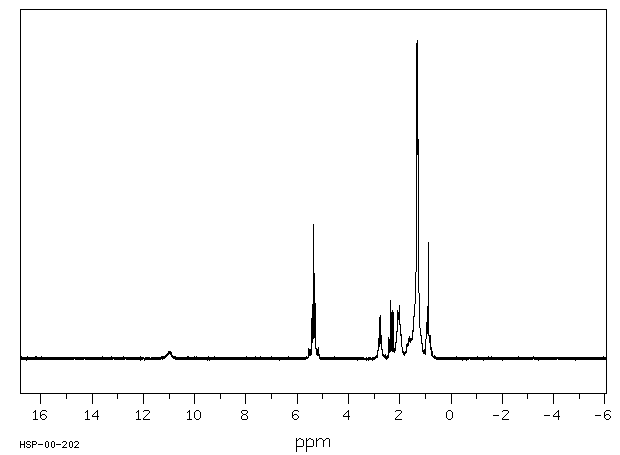
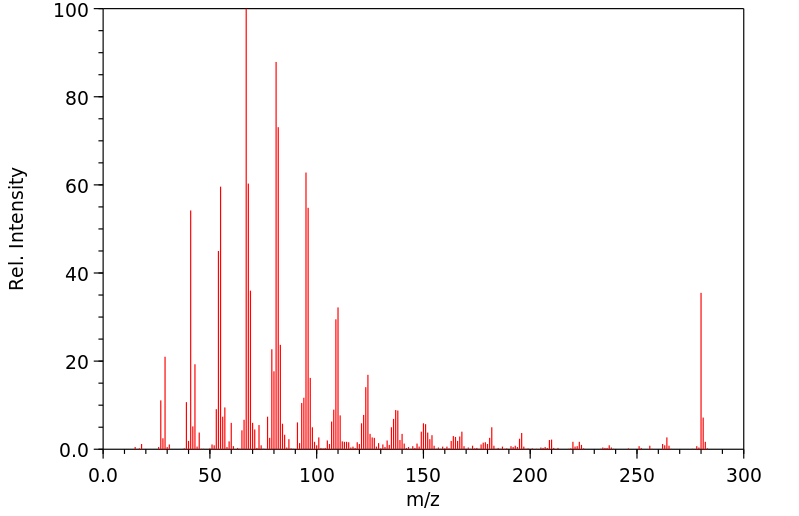
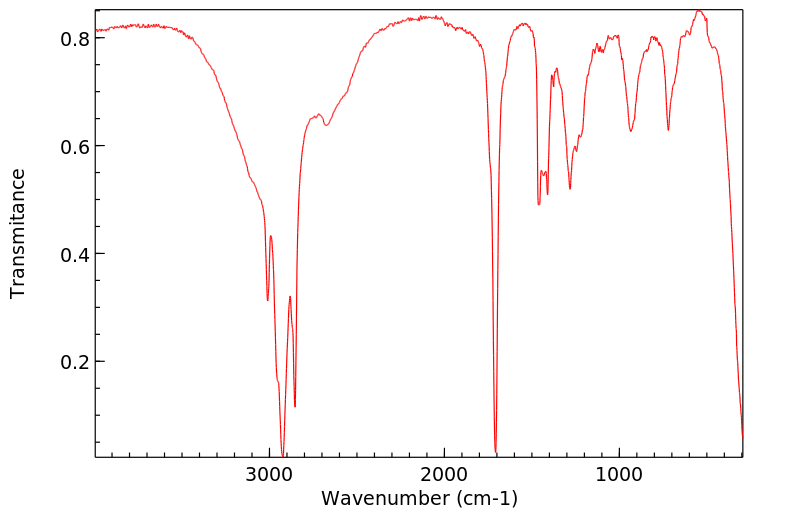
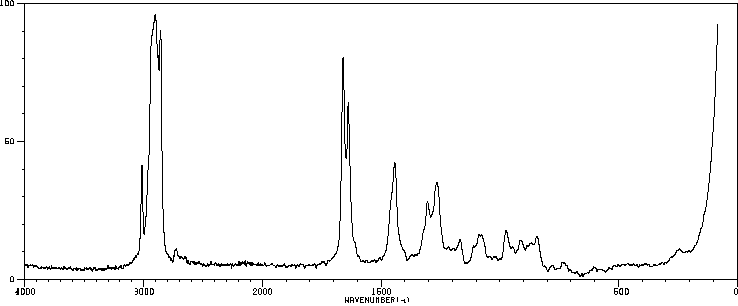
代谢
/其他毒性信息/ 口服亚油酸次级自氧化产物在大鼠中的肝毒性进行了研究,并与盐水溶液和亚油酸作为对照进行比较。次级产物组的新合成脂肪酸明显减少。肝脏中的烟酸腺嘌呤二核苷酸磷酸(NADPH)水平显著降低,而烟酸腺嘌呤二核苷酸(NADH)水平没有变化。葡萄糖-6-磷酸脱氢酶和6-磷酸葡萄糖酸脱氢酶的活性明显降低。NAD +激酶和NAD +合成酶的活性降低,而NAD +核苷酶的活性在次级产物组中增加。因此,烟酸腺嘌呤二核苷酸磷酸的消耗可以归因于两个代谢系统(烟酸腺嘌呤二核苷酸磷酸补充系统和NADP和NAD的合成系统)的抑制,导致肝脏中脂生成的减少。 /自氧化产物/
/OTHER TOXICITY INFORMATION/ The hepatotoxicity of orally administered secondary autoxidation products of linoleic acid in rats was investigated and compared to the effects following administration of a saline solution and linoleic acid as controls. The de novo synthesis of fatty acids was strongly reduced in the secondary products group. The level of nicotine adenine dinucleotide phosphate (NADPH) in the liver significantly decreased whereas that of nicotine adenine dinucleotide (NADH) did not. The activities of glucose 6-phosphate dehydrogenase and phosphogluconate dehydrogenase apparently decreased. The activities of NAD + kinase and NAD + synthetase decreased and that of NAD + nucleosidase increased in the secondary products group. Therefore the depletion of nicotine adenine dinucleotide phosphate can be attributed to the inhibition of two metabolic systems (a nicotine adenine dinucleotide phosphate-supplemental system and a synthetic system of NADP and NAD), and resulted in the reduction of lipogenesis in the liver. /Autooxidation products/
来源:Hazardous Substances Data Bank (HSDB)