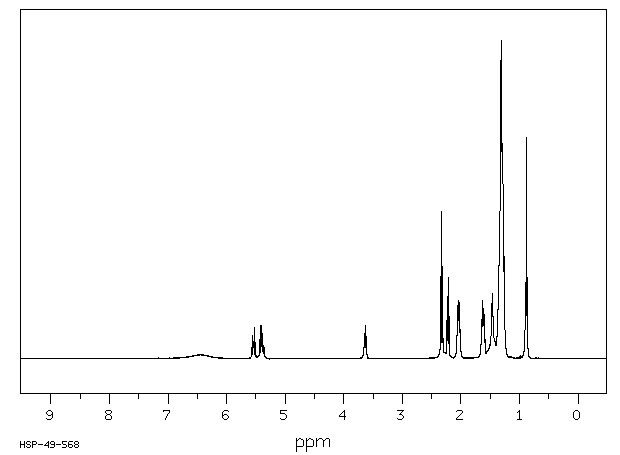
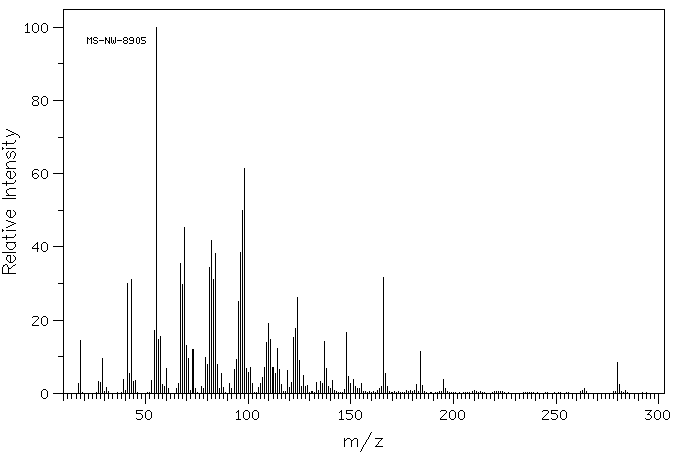
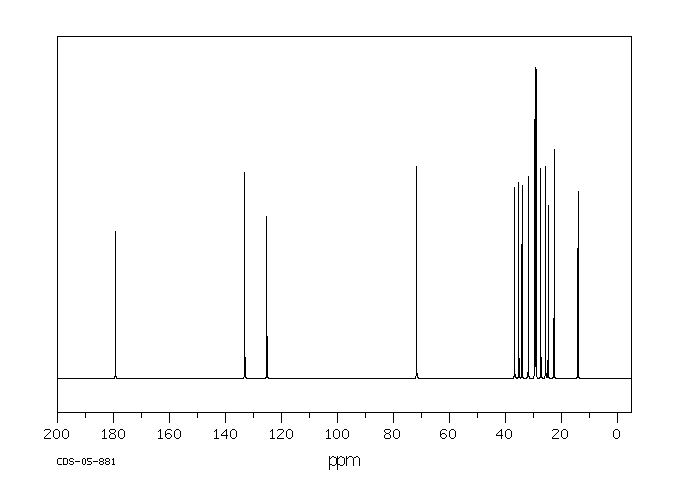
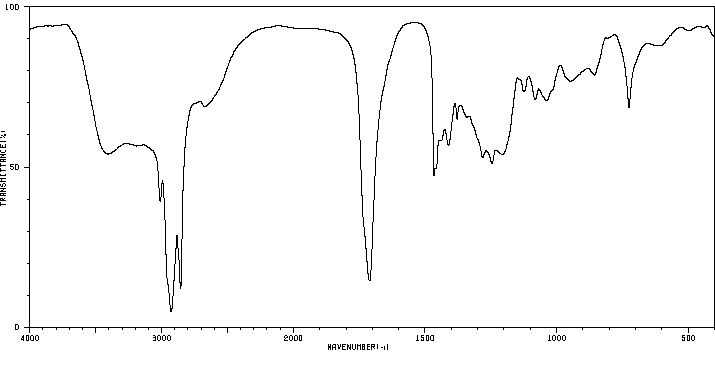
代谢
给三名健康受试者口服了
蓖麻油(10到15毫升)。在给药后2到8小时内收集尿液。尿液中排泄出了以下三种环氧二
羧酸:3,6-环氧
辛二酸;3,6-环氧
癸二酸;以及3,6-环氧
十二烷二酸。这三种
蓖麻酸代谢物在大鼠尿液中也有检测到...
来源:Hazardous Substances Data Bank (HSDB)
毒理性
蓖麻油酸(RA)与机械切削液和其他工业配方中的许多成分一样,可能是潜在的皮肤刺激物,然而关于其透过皮肤的能力却知之甚少。将3H-
蓖麻油酸与三种常用的切削液添加剂混合,分别是三嗪(TRI)、线性烷基
苯磺酸盐(
LAS)和
三乙醇胺(
TEA),然后作为
水包油(MO)或聚
乙二醇(P
EG)混合物在体外应用于惰性
硅橡胶膜和猪皮上。这些添加剂显著降低了
蓖麻油酸从配方中分配到角质层(SC)的能力,尤其是在基于P
EG的混合物中。除了
LAS之外,所有其他添加剂都产生了更偏碱性的配方(pH = 9.3-10.3)。在
硅橡胶膜和猪皮上,单独添加剂或添加剂组合显著降低了
蓖麻油酸的渗透性。这两种膜中
蓖麻油酸处置的趋势表明,混合物的相互作用更多是物理
化学性质,可能不涉及
化学诱导的
生物膜变化,这种变化可能会在接触潜在刺激物配方时被假设发生。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 立即急救:确保已经进行了充分的中毒物清除。如果患者停止呼吸,开始人工呼吸,最好使用需求阀复苏器、袋阀面罩装置或口袋面罩,按训练操作。如有必要,执行心肺复苏。立即用缓慢流动的
水冲洗受污染的眼睛。不要催吐。如果发生呕吐,让患者前倾或置于左侧(如果可能的话,头部向下)以保持呼吸道畅通,防止吸入。保持患者安静,维持正常体温。寻求医疗帮助。 /毒物A和B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 基本治疗:建立专利气道(如有需要,使用口咽或鼻咽气道)。如有必要,进行吸痰。观察呼吸不足的迹象,如有需要,协助通气。通过非循环呼吸面罩以10至15升/分钟的速度给予
氧气。监测肺
水肿,如有必要,进行治疗……。监测休克,如有必要,进行治疗……。预期癫痫发作,如有必要,进行治疗……。对于眼睛污染,立即用
水冲洗眼睛。在运输过程中,用0.9%的生理盐
水(NS)持续冲洗每只眼睛……。不要使用催吐剂。对于摄入,如果患者能够吞咽、有强烈的干呕反射且不流口
水,则用温
水冲洗口腔,并给予5毫升/千克,最多200毫升的
水进行稀释……。在去污后,用干燥的无菌敷料覆盖皮肤烧伤……。/毒药A和B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 高级治疗:对于无意识、严重肺
水肿或严重呼吸困难的病人,考虑进行口咽或鼻咽气管插管以控制气道。使用气囊面罩装置的正压通气技术可能有益。考虑使用药物治疗肺
水肿……。对于严重的支气管痉挛,考虑给予β激动剂,如
沙丁胺醇……。监测心率和必要时治疗心律失常……。开始静脉输注D5W /SRP: "保持开放",最低流量/。如果出现低血容量的迹象,使用0.9%生理盐
水(NS)或
乳酸林格氏液。对于伴有低血容量迹象的低血压,谨慎给予液体。注意液体过载的迹象……。使用
地西泮或
劳拉西泮治疗癫痫……。使用
丙美卡因氢
氯化物协助眼部冲洗……。 /Poisons A and B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/人类暴露研究/ 202名连续患者(182名女性,20名男性)患有湿疹性唇炎,他们在1996年1月至1999年12月期间参加了新加坡的接触性和职业性皮肤病的诊所,并接受了斑贴测试。女性和男性患者的平均年龄分别为31.1岁和28.8岁。斑贴测试是按照国际接触性皮炎研究小组(ICDRG)的建议进行的。29名患者(2名男性,27名女性)对
蓖麻酸产生了阳性反应。在29个反应中,有22个被认为是相关的阳性反应。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
蓖麻油酸或甲基
蓖麻油酸通过胃管给药给体重至少400克的雄性大鼠,这些大鼠的胸导管插有管子。收集了48小时的淋巴液,然后提取并分离出各种脂质类别。结果表明,
蓖麻油酸存在于
甘油三酯、
甘油二酯、
甘油一酯和游离
脂肪酸部分。
蓖麻油酸的最大吸收发生在给药后30分钟内。淋巴脂质中的
磷脂或
胆固醇酯部分没有
蓖麻油酸。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
为了提高
蓖麻酸在体内的荧光性,本研究向
蓖麻酸中添加了甲基
安息香酸或甲基胆
蒽,比例为1:99。实验物质被温和地涂抹在已经剃毛的皮肤上。在涂抹后不同时间点进行了活组织切片检查。使用带有石英聚光镜的斯宾塞显微镜观察了样本。
蓖麻酸主要保留在表皮的外层。在涂抹后2小时取的活检中,几乎没有证据表明有更深的渗透。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
使用Bronaugh流通扩散
细胞系统,评估了标记有放射性同位素(^3H)的ricinoleic酸(比活性=20.0 mCi/mmol)混合物通过猪皮膜或
硅橡胶(聚二甲基
硅氧烷)膜的经皮吸收。在
水中制备了含有5%矿物油或5%P
EG 200的^3H标记的ricinoleic酸(5%)混合物。其他^3H标记的ricinoleic酸混合物采用了以下三种常用的切削液添加剂:三嗪、线性烷基
苯磺酸盐和
三乙醇胺。在局部暴露8小时后,ricinoleic酸的吸收(基于受体液中回收的量)在
硅橡胶膜中从1%到13%,在猪皮膜中从0.1%到0.3%。对于大多数混合物,ricinoleic酸的吸收峰出现在3小时内。在两种膜中,含有P
EG的控制混合物与ricinoleic酸的最高峰浓度相关。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
调查人员在两个实验中研究了在大鼠被喂食麻酸后在脂肪库中羟基酸的积累情况。在第一个实验中,成年雄性大鼠(数量、品系和体重未说明)被喂食麻酸(5%乳液,20毫升)7天。在第二个实验中,动物被喂食了27天。从脂肪组织中提取脂质后,通过
水解得到一种
脂肪酸混合物。气液色谱图显示,与麻酸链长较短的以下羟基
脂肪酸含量显著:10-羟基
十六碳烯酸(实验1:占总
脂肪酸的0.60%;实验2:占总
脂肪酸的0.33%),8-羟基十四碳烯酸(实验1:占总
脂肪酸的0.03%;实验2:占总
脂肪酸的0.08%),以及6-羟基十二碳烯酸(实验2:占总
脂肪酸的0.03%)。麻酸在实验1中占总
脂肪酸的0.51%,在实验2中占总
脂肪酸的3.85%。
来源:Hazardous Substances Data Bank (HSDB)