蔗糖 | 57-50-1
中文名称
蔗糖
中文别名
D-(+)-蔗糖;蔗糖丁烯酯;β-D-呋喃果糖基-α-D-吡喃葡萄苷
英文名称
Sucrose
英文别名
sugar;saccharose;D-saccharose;β-D-Fruf-(2↔1)-α-D-Glcp;(2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
CAS
57-50-1
化学式
C12H22O11
mdl
MFCD00006626
分子量
342.3
InChiKey
CZMRCDWAGMRECN-UGDNZRGBSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:185-187 °C (lit.)
-
比旋光度:67 º (c=26, in water 25 ºC)
-
沸点:397.76°C (rough estimate)
-
密度:1.5805
-
闪点:93.3°C
-
溶解度:在水中的溶解度500 mg/mL
-
最大波长(λmax):λ: 260 nm Amax: 0.11λ: 280 nm Amax: 0.08
-
暴露限值:ACGIH: TWA 10 mg/m3OSHA: TWA 15 mg/m3; TWA 5 mg/m3NIOSH: TWA 10 mg/m3; TWA 5 mg/m3
-
介电常数:3.3(Ambient)
-
LogP:-4.492 (est)
-
物理描述:Sucrose appears as white odorless crystalline or powdery solid. Denser than water.
-
颜色/状态:Monoclinic sphenoidal crystals, crystalline masses, blocks, or powder
-
气味:Characteristic caramel
-
味道:Sweet
-
蒸汽压力:0 mm Hg (approx) (NIOSH, 2016)
-
稳定性/保质期:
STABLE IN AIR
-
分解:186 °C
-
燃烧热:-1.35X10+6 cal/mol
-
表面张力:71-75 mN/m @ 1-0.6 mol/l
-
折光率:INDEX OF REFRACTION: 1.5376; SADTLER REFERENCE NUMBER: 8659 (IR, PRISM), 563 (IR, GRATING); SPECIFIC OPTICAL ROTATION: +66.37 @ 20 °C/D (WATER)
-
Caco2细胞的药物渗透性:-5.77
-
解离常数:pKa= 12.62
-
碰撞截面:174 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):-3.7
-
重原子数:23
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:190
-
氢给体数:8
-
氢受体数:11
ADMET
毒理性
A4:不能归类为人类致癌物。
A4: Not classifiable as a human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
这种物质可以通过吸入和摄入被身体吸收。
The substance can be absorbed into the body by inhalation and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,皮肤和/或眼睛接触
inhalation, skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
眼睛、皮肤、上呼吸道刺激;咳嗽
irritation eyes, skin, upper respiratory system; cough
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
咳嗽。
Cough.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 10 mg/m3 (total)
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
WGK Germany:2
-
海关编码:1701991090
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:WN6500000
SDS
制备方法与用途
水中溶解度(g/100ml):不同温度(℃)时每100毫升水中的溶解克数:
- 181.9g/0℃
- 190.6g/10℃
- 201.9g/20℃
- 216.7g/30℃
- 235.6g/40℃
- 259.6g/50℃
- 288.8g/60℃
- 323.7g/70℃
- 365.1g/80℃
- 414.9g/90℃
- 476g/100℃
毒性:GRAS(FDA,§184.1854,2000)。 LD₅₀为29700mg/kg(大鼠,经口摄入)。
使用限量:不作限制性规定(FDA,§184.1854,2000)。
化学性质:白色结晶性无臭固体,具有甜味。相对密度(d₂⁴)为1.587,熔点为170~186℃(分解)。易溶于水,不溶于乙醚。
用途:营养性甜味剂;成型和组织结构剂。 蔗糖是最普通的食品添加剂,也用于制柠檬酸、焦糖、转化糖、透明肥皂等。在高浓度时能抑制细菌生长,在医药上可作防腐剂、抗氧剂的药片赋形剂等。
1980年世界原糖产量为8710万吨,其中蔗糖5200万吨,甜菜糖3500万吨。来自甘蔗和甜菜的蔗糖受自然条件限制发展缓慢,全球缺糖情况较为严重。各种特殊需求促进了新型甜料的研究和发展,目前已经找到了许多天然甜料和合成甜料。
试剂蔗糖用于1-萘酚的测定,也用于钙、镁分离及生物培养基的制备。细胞培养级蔗糖可用于蔗糖梯度纯化病毒和蛋白质;植物细胞培养级用于植物细胞中的冷冻保护;分子生物学级用于不连续蔗糖密度梯度的等密度离心法,如从膜结合核糖体中分离mRNA。
多种物质保存液和辅助液、生物培养基。分离钙和镁。测定1-萘酚。络合滴定硼的掩蔽剂。有机微量分析测定碳、氢和氧的标准。
生产方法:蔗糖大量来自甘蔗糖和甜菜,甘蔗含蔗糖约15-20%,甜菜含10-17%。其他各种果实、种子、叶、花、根中也有不同的含量。将甘蔗榨出法液或将甜菜切片用水提出糖汁,用石灰澄清法或兼用亚硫酸饱充法除去糖汁中的杂质,过滤后将滤液真空蒸浓,再结晶分离而得粗糖,再经脱色、重结晶而得精糖。
生产方法:由甘蔗或甜菜经压榨或渗滤后澄清、蒸发、结晶而得。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 D-水苏糖 stachyose 470-55-3 C24H42O21 666.585 棉子糖 D-raffinose 512-69-6 C18H32O16 504.442 —— α-D-glucopyranosyl-(1→2)-[α-D-glucopyranosyl-(1→6)]-β-D-fructofuranoside —— C18H32O16 504.442 6-O-alpha-D-吡喃半乳糖基-beta-D-果糖呋喃糖基 alpha-D-吡喃葡萄糖苷 α-D-glucopyranosyl-(1->2)-<α-D-galactopyranosyl-(1->6)->-β-D-fructofuranoside 470-57-5 C18H32O16 504.442 蔗果三糖 1-kestose 470-69-9 C18H32O16 504.442 —— 2,1':4,6-di-O-isopropylidene sucrose 67909-39-1 C18H30O11 422.43 —— Isomaltulose —— C12H22O11 342.3 —— 6-O-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl]-β-D-fructofuranosyl-(2->1)-α-D-glucopyranoside 1053246-35-7 C30H50O12 602.72 海藻糖 TREHALOSE 99-20-7 C12H22O11 342.3 蔗糖八乙酸酯 sucrose octaacetate 126-14-7 C28H38O19 678.598 蔗糖棕榈酸酯 saccharose monopalmitate 39300-95-3 C28H52O12 580.714 异麦芽酮糖醇 Isomalt 64519-82-0 C12H24O11 344.316 异麦芽糖醇 isomaltitol 534-73-6 C12H24O11 344.316 —— D-isomaltose 24822-33-1 C12H22O11 342.3 —— isomaltotriose 455-50-5 C18H32O16 504.442 —— Cellobiose 13360-52-6 C12H22O11 342.3 —— maltose —— C12H22O11 342.3 糊精 Dextrin 9004-53-9 C18H32O16 504.4 D-(+)-纤维二糖 Maltose 133-99-3 C12H22O11 342.3 —— glansreginin B 860267-53-4 C24H38O15 566.557 麦芽糖 D-maltose 69-79-4 C12H22O11 342.3 D-果糖 D-fructose 470-23-5 C6H12O6 180.158 —— D-fructose 10247-46-8 C6H12O6 180.158 —— 2'-O-acetyl-3-O-(E)-p-coumaroylsucrose —— C23H30O14 530.483 —— β-D-(1-O-acetyl-3,6-O-p-E-dicoumaroyl)-fructofuranosyl-α-D-(4'-O-acetyl-2'-O-p-E-coumaroyl)-glucopyranoside 1140954-78-4 C43H44O19 864.811 —— 3',6'-di-O-acetyl-3-O-(E)-p-coumaroylsucrose —— C25H32O15 572.52 —— mumeose C 1446205-86-2 C27H34O16 614.557 —— 4,3',6'-tri-O-acetyl-3-O-(E)-p-coumaroylsucrose —— C27H34O16 614.557 —— 4,2',3'-tri-O-acetyl-3-O-(E)-p-coumaroylsucrose —— C27H34O16 614.557 —— 3',4',6'-tri-O-acetyl-3-O-(E)-p-coumaroylsucrose —— C27H34O16 614.557 —— 6,3',4',6'-tetra-O-acetyl-3-O-(E)-p-coumaroylsucrose —— C29H36O17 656.595 —— mumeose E 1392307-47-9 C31H38O18 698.632 —— mumeose D 1392307-46-8 C31H38O18 698.632 —— 1,4,2',3',4',6'-hexa-O-acetyl-3-O-(E)-p-coumaroylsucrose —— C33H40O19 740.669 1-O-Alpha-D-吡喃葡萄糖基-D-呋喃果糖 1-O-α-D-glucopyranosyl-β-D-fructose 51411-23-5 C12H22O11 342.3 a-无水葡萄糖酯 alpha-D-glucopyranose 492-62-6 C6H12O6 180.158 alpha-D-半乳糖 α-D-galactopyranose 3646-73-9 C6H12O6 180.158 D-葡萄糖 D-glu 2280-44-6 C6H12O6 180.158 —— (3-O-feruloyl)-β-D-fructofuranosyl-(2→1)-(6-O-feruloyl)-α-D-glucopyranoside —— C32H38O17 694.644 —— 3,6-diferuloyl-6′-acetylsucrose 107647-21-2 C34H40O18 736.681 a-D-吡喃葡萄糖苷,3,6-二-O-[(2E)-3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯-1-基]-b-D-果呋喃糖基 3,6-diferuloylsucrose 107647-20-1 C32H38O17 694.644 —— (3,6-di-O-Z-feruloyl)-β-D-fructofuranosyl-(1->2)-α-D-glucopyranoside 1235513-34-4 C32H38O17 694.644 —— integrisides A —— C34H42O19 754.696 —— (3,6-O-disinapoyl)-β-D-fructofuranosyl-(2→1)-(6-O-sinapoyl)-α-D-glucopyranoside —— C45H52O23 960.894 —— 1,3-di-(6-O-sucrose)-1,1,3,3-tetrabutyl-distannoxane —— C40H78O23Sn2 1164.47 —— (3-O-feruloyl-4-O-sinapoyl)-β-D-fructofuranosyl-(2→1)-(6-O-sinapoyl)-α-D-glucopyranoside —— C44H50O22 930.868 2,3,4,6-四-o-苄基-D-吡喃葡萄糖 2,3,4,6-tetra-O-benzyl-D-glucopyranose 6564-72-3 C34H36O6 540.656 —— glucose 6-phosphate 15209-12-8 C6H13O9P 260.138 - 1
- 2
- 3
- 4
- 5
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— β-D-fructofuranosyl-β-L-galactopyranoside —— C12H22O11 342.3 —— β-D-fructofuranosyl-β-L-glycopyranoside —— C12H22O11 342.3 阿洛蔗糖 allosucrose 4217-76-9 C12H22O11 342.3 —— sucrose —— C12H22O11 342.3 (2R,3S,4S,5S,6R)-2-[(2R,3S,4S,5R)-3,4-二羟基-2,5-二(羟基甲基)四氢呋喃-2-基]氧基-6-(羟基甲基)四氢吡喃-3,4,5-三醇 β-D-fructofuranosyl α-D-mannopyranoside 79324-70-2 C12H22O11 342.3 —— β-D-fructofuranosyl-α-D-fucopyranoside —— C12H22O10 326.301 —— β-D-fructofuranosyl-β-L-fucopyranoside —— C12H22O10 326.301 —— 6'-deoxysucrose 99281-34-2 C12H22O10 326.301 —— 2G-deoxysucrose 6852-68-2 C12H22O10 326.301 —— β-D-fructofuranosyl-α-D-xylopyranoside —— C11H20O10 312.274 —— β-D-fructofuranosyl-β-L-xylopyranoside —— C11H20O10 312.274 棉子糖 D-raffinose 512-69-6 C18H32O16 504.442 D-水苏糖 stachyose 470-55-3 C24H42O21 666.585 —— rafinose —— C18H32O16 504.442 —— raffinose 21291-36-1 C18H32O16 504.442 龙胆三糖 theanderose 25954-44-3 C18H32O16 504.442 —— 6-kestose 562-68-5 C18H32O16 504.442 —— Iso-neokestose 138809-77-5 C18H32O16 504.442 —— α-D-glucopyranosyl-(1->2)-<β-D-galactopyranosyl-(1->6)>-β-D-fructofuranoside 41110-70-7 C18H32O16 504.442 —— α-D-galactopyranosyl β-D-fructofuranosyl-(2-6)-β-D-fructofuranoside 96480-25-0 C18H32O16 504.442 6-O-alpha-D-吡喃半乳糖基-beta-D-果糖呋喃糖基 alpha-D-吡喃葡萄糖苷 α-D-glucopyranosyl-(1->2)-<α-D-galactopyranosyl-(1->6)->-β-D-fructofuranoside 470-57-5 C18H32O16 504.442 —— phlein hexasaccharide 137855-42-6 C36H62O31 990.87 —— α-D-fructofuranosyl-(2→6)-β-D-fructofuranosyl-(2→1)-α-D-glucopyranoside 138809-76-4 C18H32O16 504.442 —— neokestose 3688-75-3 C18H32O16 504.442 —— α-D-glucopyranosyl-(1→2)-[α-D-glucopyranosyl-(1→6)]-β-D-fructofuranoside —— C18H32O16 504.442 —— 6'-O-methylsucrose 59001-35-3 C13H24O11 356.327 —— 6,6-Kestotetraose 119187-86-9 C24H42O21 666.6 —— 6-O-hexadecylsucrose 187169-00-2 C28H54O11 566.73 —— decyl(-6)Glc(a1-2b)Fruf 1447967-67-0 C22H42O11 482.569 —— (2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(dodecoxymethyl)oxane-3,4,5-triol 13047-15-9 C24H46O11 510.623 —— 6'-O-hexadecylsucrose 1261272-27-8 C28H54O11 566.73 —— 6-O-(2RS-hyydroxydodecyl)sucrose —— C24H46O12 526.622 —— 3G-α-D-Glucosyl-sucrose —— C18H32O16 504.442 —— β-D-galactopyranosyl-(1->3)-α-D-glucopyranosyl-(1<->2)-β-D-fructofuranoside —— C18H32O16 504.442 —— 6'-O-(2RS-hydroxydodecyl)sucrose —— C24H46O12 526.622 —— O-α-D-galactopyranosyl-(1->3)-O-α-D-glucopyranosyl-(1->2)-β-D-fructofuranoside 2650-46-6 C18H32O16 504.442 —— 1'-O-methylsucrose 77895-08-0 C13H24O11 356.327 低聚乳果糖 lactosucrose 87419-56-5 C18H32O16 504.442 吡喃葡糖基蔗糖 O-α-D-glucopyranosyl-(1->4)-α-D-glucopyranosyl-(1->2)-β-D-fructofuranoside 13101-54-7 C18H32O16 504.442 —— β-Fruf-(2->1)-α-Glcp-(4<-1)-α-Galp —— C18H32O16 504.442 —— β-D-glucopyranosyl-(1->4)-α-D-glucopyranosyl-(1->2)-β-D-fructofuranoside —— C18H32O16 504.442 —— α-D-glucopyranosyl-(1->4)-α-D-glucopyranosyl-(1->4)-α-D-glucopyranosyl-(1->2)-β-D-fructofuranoside —— C24H42O21 666.585 —— α-D-Glcp-(1->4)-α-D-Glcp-(1->4)-α-D-Glcp-(1->4)-Glcp-(1<->2)-β-D-Fruf —— C30H52O26 828.727 —— 3-O-(2RS-hydroxydodecyl)sucrose —— C24H46O12 526.622 —— (2S,3R,4S,5R,6R)-2-(((2R,3R,4S,5S,6R)-2-(((2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-yl)oxy)-4,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-3-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol 1004760-17-1 C18H32O16 504.442 —— neoketose 894412-30-7 C18H32O16 504.442 —— 4-O-(2RS-hydroxydodecyl)sucrose —— C24H46O12 526.622 蔗果三糖 1-kestose 470-69-9 C18H32O16 504.442 蔗果四糖 nystose 13133-07-8 C24H42O21 666.585 蔗果五糖 1-fructofuranosylnystose 59432-60-9 C30H52O26 828.727 —— α-D-fructofuranosyl-(2→1)-β-D-fructofuranosyl-(2→1)-α-D-glucopyranoside 138809-75-3 C18H32O16 504.442 —— Bifurcose 3568-31-8 C24H42O21 666.6 —— 1'-O-(2RS-hydroxydodecyl)sucrose —— C24H46O12 526.622 —— β-D-galactopyranosyl-(1→4)-β-D-fructofuranosyl-(2→1)-α-D-glucopyranoside 4955-91-3 C18H32O16 504.442 —— (4aR,6R,7R,8R,8aS)-6-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-2-methyl-2-undecyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxine-7,8-diol 187879-59-0 C25H46O11 522.634 D-(+)-松三糖水合物 melezitose 597-12-6 C18H32O16 504.442 —— octa-O-methyl-sucrose 5346-73-6 C20H38O11 454.515 —— O-β-D-glucopyranosyl-(1→3)-β-D-fructofuranosyl α-D-glucopyranoside —— C18H32O16 504.442 —— 1',2,3,3',4,4'-hexa-O-methylsucrose 63734-21-4 C18H34O11 426.461 —— 3'-O-(2RS-hydroxydodecyl)sucrose —— C24H46O12 526.622 —— 2,1':4,6-di-O-isopropylidene sucrose 67909-39-1 C18H30O11 422.43 —— 2,1':4,6-Di-O-isopropylidene-6'-O-(1-methyl-1-methoxyethyl)sucrose —— C22H38O12 494.537 —— sucrose-6-propionate 70284-35-4 C15H26O12 398.364 —— α-D-fructofuranosyl-(2->6)-D-glucopyranose 29585-40-8 C12H22O11 342.3 —— 6'-O-butanoyl-sucrose —— C16H28O12 412.391 八(O-氰基乙基)蔗糖 1',2,3,3',4',6'-Octa-O-(2-cyanoethyl)sucrose 18304-13-7 C36H46N8O11 766.808 —— sucrose 1428146-77-3 C92H150O11 1432.2 —— 4,6-O-methoxyethylidenesucrose —— C15H26O12 398.364 —— β-D-fructofuranosyl-(O6-hexanoyl-α-D-glucopyranoside) 70284-39-8 C18H32O12 440.445 —— sucrose 6-O-isobutyrate —— C16H28O12 412.391 —— octa-O-allylsucrose 14699-90-2 C36H54O11 662.818 —— sucrose-6-glutarate 128489-96-3 C17H28O14 456.401 —— 1,2-O-isopropylidene-β-D-fructopyranose 66900-93-4 C9H16O6 220.222 —— capric acid sucrose monoester 14533-07-4 C22H40O12 496.552 —— 6-O-sucrose monolaurate 13039-40-2 C24H44O12 524.606 —— β-D-fructofuranosyl 6-O-myristyl-α-D-glucopyranoside 20881-07-6 C26H48O12 552.66 蔗糖棕榈酸酯 β-D-fructofuranosyl 6-O-palmityl-α-D-glucopyranoside 13039-41-3 C28H52O12 580.714 —— β-D-fructofuranosyl 6-O-stearyl-α-D-glucopyranoside 13039-42-4 C30H56O12 608.767 —— caprylic acid sucrose monoester 13039-39-9 C20H36O12 468.499 —— 6-O-docosanoylsucrose —— C34H64O12 664.875 —— n-pentyl β-D-fructofuranoside 195379-74-9 C11H22O6 250.292 正丁基-Β-D-呋喃果糖苷 β-D-fructofuranose 2-butyl ether 80971-60-4 C10H20O6 236.265 —— Isomaltulose —— C12H22O11 342.3 —— isomaltulose 15132-06-6 C12H22O11 342.3 —— sucrose 6'-O-monolaurate 20881-05-4 C24H44O12 524.606 —— 6,6'-di-O-stearoylsucrose 657372-37-7 C48H90O13 875.235 —— sucrose-6,6'-di-O-laurate 20881-06-5 C36H66O13 706.912 —— 6'-sucrose monooctanoate 136152-82-4 C20H36O12 468.499 —— 6,6'-di-O-octanoylsucrose 58064-47-4 C28H50O13 594.697 —— 6,6'-di-O-hexadecanoylsucrose 20881-09-8 C44H82O13 819.127 —— 6'-O-palmitoylsucrose 24516-45-8 C28H52O12 580.714 —— 6'-O-myristoylsucrose 182750-19-2 C26H48O12 552.66 —— 6,6'-di-O-decanoylsucrose —— C32H58O13 650.805 —— stearic acid sucrose ester 136152-87-9 C30H56O12 608.767 —— 1'-O-monobutyrylsucrose 111933-83-6 C16H28O12 412.391 —— octa-O-methallylsucrose —— C44H70O11 775.033 —— 6-monoamine-monodeoxysucrose 82540-70-3 C12H23NO10 341.315 —— α-D-fructofuranose β-D-fructopyranose 1,2':2,1'-dianhydride 97415-70-8 C12H20O10 324.285 乙基 beta-D-呋喃果糖苷 ethyl β-D-fructofuranoside 1820-84-4 C8H16O6 208.211 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:Supported copper catalysts for highly efficient hydrogenation of biomass-derived levulinic acid and γ-valerolactone摘要:将纤维素衍生的乙酰丙酸直接转化为γ-戊内酯,再将γ-戊内酯转化为1,4-戊二醇,通过支持铜催化剂进行化学选择性加氢。DOI:10.1039/c5gc01454a
-
作为产物:参考文献:名称:洪冈三角龙的四种新的蔗糖酸取代蔗糖酸二酯,它们具有对octamin-1的抑制活性。摘要:由于存在于天然来源中很少发现的环丁烷环和大环蔗糖二酯部分,因此杜鹃酸蔗糖二酯类似物具有令人感兴趣的化学结构。本文描述了分离和取代truxinic酸,trigohonbanosides AD(四个新的蔗糖二酯结构解析1 - 4)中,从叶三宝木属honbaensis。通过HR-ESI-MS,NMR和CD光谱法阐明了它们的化学结构。在30μM的浓度,化合物1 - 4 27.7±1.10%的抑制百分数分别适度抑制ANO-1活性,35.6±0.92%,43.7±1.61%,和40.8±1.25%。DOI:10.1016/j.bioorg.2020.104058
-
作为试剂:描述:(S)-2-ethynyl-glycerol phosphate 在 copper(ll) sulfate pentahydrate 、 manganese(II) chloride tetrahydrate 、 三乙醇胺 、 双(2-羟乙基)氨基(三羟甲基)甲烷 、 catalase from Corynebacterium glutamicum 、 丙酸 、 DERARd4BB 、 galactose oxidase GOaseRd17BB 、 PMRd12BB 、 PNPRd5BB 、 SRRd3BB 、 potassium hydroxide 、 horseradish peroxidase 、 蔗糖 作用下, 以 水 为溶剂, 5.0~35.0 ℃ 、293.86 kPa 条件下, 反应 41.5h, 生成 2-脱氧-4-c-乙炔-2-氟腺苷酸参考文献:名称:开发 Islatravir 生产路线的生物催化有氧氧化摘要:生物催化氧化有潜力解决许多合成挑战,能够选择性合成手性中间体,例如羰基化合物、醇或胺。使用氧依赖性酶可以显着减少生产规模氧化还原转化的环境足迹。在这里,作为抗 HIV 研究药物 islatravir ( 1 ) 生物催化级联的一部分,我们描述了使用进化的半乳糖氧化酶递送 ( R )-乙炔基甘油醛-3-磷酸 ( 3 ) 的有氧氧化过程的开发。集成的酶和反应工程对于在中试规模(>20 kg,90% 产率)中实现稳健、高产的氧化至关重要。DOI:10.1021/acs.oprd.4c00075
文献信息
-
Simple one-pot regioselective 6-O-phosphorylation of carbohydrates and trehalose desymmetrization作者:A. Abragam Joseph、Chun-Wei Chang、Cheng-Chung WangDOI:10.1039/c3cc47180b日期:——Biologically essential carbohydrate 6-phosphates, especially trehalose 6-phosphate, can be synthesized easily in excellent overall yields in 2 steps involving minimum protecting group manipulations. We can cleave the diphenylphosphate group for further synthetic objectives.
-
Targeted Synthesis of 3,3′-, 3,4′- and 3,6′-Phenylpropanoid Sucrose Esters作者:Wong Pooi Wen Kathy、Li Lin Ong、Surabhi Devaraj、Duc Thinh Khong、Zaher M. A. JudehDOI:10.3390/molecules27020535日期:——orthogonal strategy for the precise synthesis of 3,3′-, 3,4′-, and 3,6′-phenylpropanoid sucrose esters (PSEs). The strategy relies on carefully selected protecting groups and deprotecting agents, taking into consideration the reactivity of the four free hydroxyl groups of the key starting material: di-isopropylidene sucrose 2. The synthetic strategy is general, and potentially applies to the preparation of
-
Convenient Syntheses of 2,3,4,6-Tetra-O-AlkylatedD-Glucose andD-Galactose作者:Ludwig Käsbeck、Horst KesslerDOI:10.1002/jlac.199719970124日期:1997.1Convenient syntheses of the 2,3,4,6-tetra-O-benzylated and -allylated D-glucopyranoses 1 and 2 and the corresponding D-galactopyranoses 3 and 4 are described. The D-glucose derivatives 1 and 2 were obtained from inexpensive sucrose by peralkylation and subsequent acid hydrolysis. In this reaction sequence an alkylated D-fructofuranosyl cation is generated which was trapped by different nucleophiles描述了2,3,4,6-四-O-苄基化和烯丙基化的D-吡喃葡萄糖1和2以及相应的D-吡喃半乳糖3和4的方便的合成。D-葡萄糖衍生物1和2是从廉价的蔗糖中通过过烷基化和随后的酸水解获得的。在该反应序列中,产生烷基化的D-果糖呋喃糖基阳离子,该阳离子被不同的亲核试剂捕获,得到4-苄氧基亚甲基糠醛(8)和烷基化的D-果糖苷7、8和10。另一方面,2,3,4,6-四-O-苄基-D-半乳糖3通过用N-溴代琥珀酰亚胺水溶液氧化裂解乙基2,3,4,6-四-O-苄基-1-硫代-α,β-D-吡喃半乳糖苷(11)来合成。通过对甲氧基苄基β-D-葡糖苷制备2,3,4,6-四-O-烯丙基-D-吡喃葡萄糖苷(2)的替代途径吸引力较小。然而,被用于的2,3,4,6-四-合成此路线ö -allyl- d半乳糖(4)。
-
Reaction of cyclic ketals with ceric ammonium nitrate in acetonitrile/water作者:Emiliano Manzo、Gaspare Barone、Emiliano Bedini、Alfonso Iadonisi、Lorenzo Mangoni、Michelangelo ParrilliDOI:10.1016/s0040-4020(01)01006-7日期:2002.1performing the hydrolysis of cyclic acetals and ketals under basic or mildly alkaline conditions by ceric ammonium nitrate (CAN) in MeCN/H2O or in 10% aq. methanol is rejected. However a role for Ce(IV) as Lewis acid is evidenced at pH 4.4. Under these conditions, which allow hydrolysis of cyclic ketals leaving glycosidic bonds unaltered, a selectivity of ketal cleavage due to steric hindrance is also
-
Simultaneous Conversion of C <sub>5</sub> and C <sub>6</sub> Sugars into Methyl Levulinate with the Addition of 1,3,5‐Trioxane作者:Xilei Lyu、Zihao Zhang、Francis Okejiri、Hao Chen、Mai Xu、Xujie Chen、Shuguang Deng、Xiuyang LuDOI:10.1002/cssc.201902096日期:2019.10.8The simultaneous conversion of C5 and C6 mixed sugars into methyl levulinate (MLE) has emerged as a versatile strategy to eliminate costly separation steps. However, the traditional upgrading of C5 sugars into MLE is very complex as it requires both acid-catalyzed and hydrogenation processes. This study concerns the development of a one-pot, hydrogenation-free conversion of C5 sugars into MLE over different将C5和C6混合糖同时转化为乙酰丙酸甲酯(MLE)已成为一种消除昂贵分离步骤的通用策略。但是,传统的将C5糖升级为MLE的过程非常复杂,因为它需要酸催化过程和加氢过程。这项研究涉及在接近关键的甲醇条件下借助1,3,5-三恶烷在不同的酸催化剂上将一锅一锅的无加氢的C5糖转化为MLE的进展。对于不添加1,3,5-三恶烷的沸石上的C5糖转化而言,由于氢化活性低,MLE收率非常低。1,3,5-三恶烷的添加通过提供不包括氢化步骤的替代转化途径,显着提高了MLE收率。直接比较五种不同沸石的催化性能表明,具有高路易斯酸位和布朗斯台德酸位密度的Hβ沸石可提供最高的MLE收率。加入1,3,5-三恶烷,糠醛衍生物和甲醛的羟甲基化是关键步骤。值得注意的是,当完全优化反应条件时,由Hβ沸石催化的C5和C6糖的同时转化可以实现高达50.4%的MLE收率。而且,Hβ沸石催化剂可以重复使用至少五次,而性能没有显着变化。
表征谱图
-
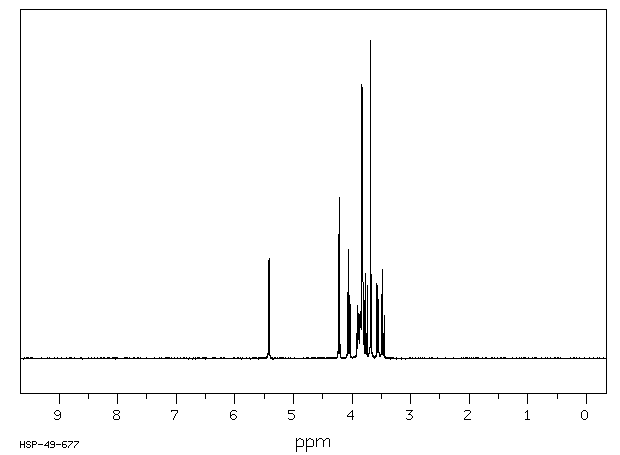
氢谱1HNMR
-
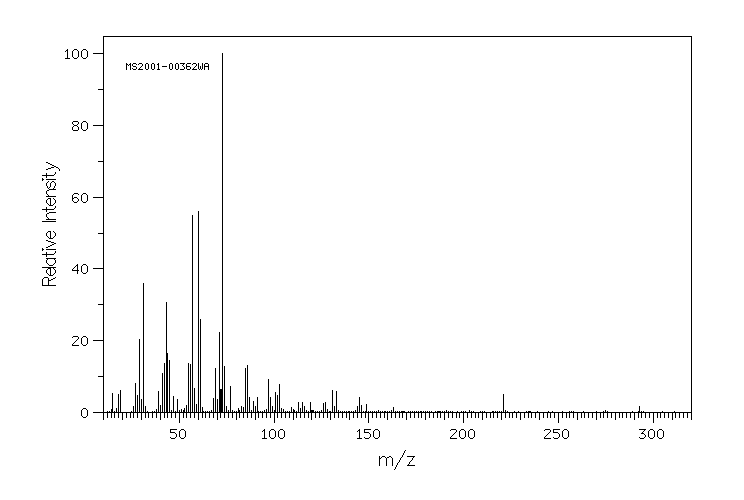
质谱MS
-
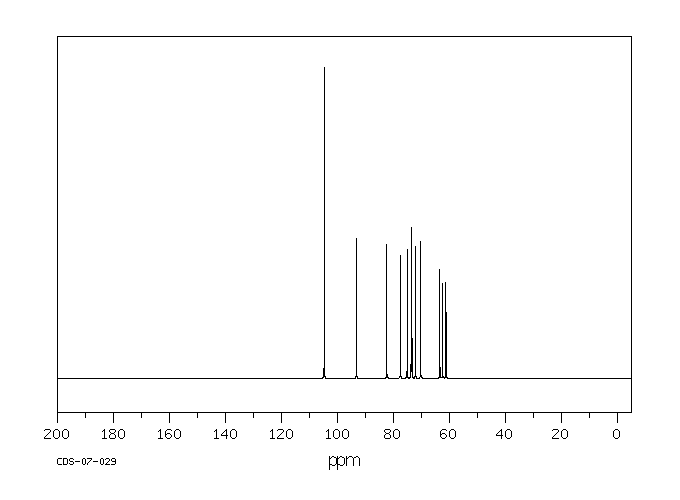
碳谱13CNMR
-
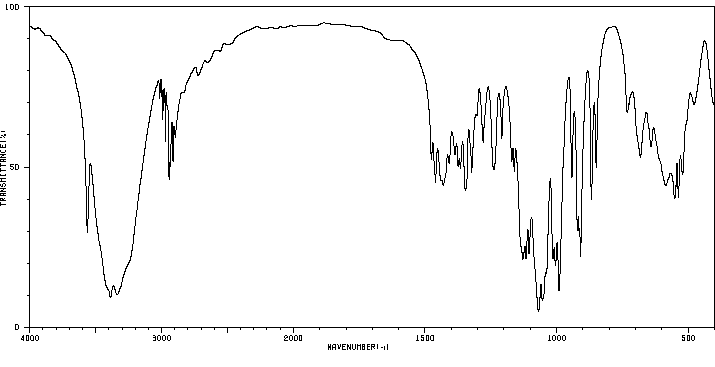
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷