视黄醛 | 116-31-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:61-63°C
-
沸点:366.92°C (rough estimate)
-
密度:1.0083 (rough estimate)
-
溶解度:可溶于氯仿(少许)、乙酸乙酯(少许)、甲醇(少许)
-
物理描述:Solid
-
碰撞截面:170.1 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
-
保留指数:2466;2466
计算性质
-
辛醇/水分配系数(LogP):6.2
-
重原子数:21
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.45
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
危险品标志:Xn
-
安全说明:S22,S36/37
-
危险类别码:R22,R38
-
WGK Germany:3
-
海关编码:2912299000
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:VH6407000
-
危险标志:GHS07
-
危险性描述:H302,H315
-
危险性防范说明:P280
-
储存条件:-20°C
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : all trans-Retinal
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 116-31-4
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 4), H302
Skin irritation (Category 2), H315
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xn Harmful R22, R38
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Warning
Hazard statement(s)
H302 Harmful if swallowed.
H315 Causes skin irritation.
Precautionary statement(s) none
Supplemental Hazard none
Statements
Safety data sheet available on request.
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Synonyms : Vitamin A aldehyde
Formula : C20H28O
Molecular Weight : 284,44 g/mol
CAS-No. : 116-31-4
EC-No. : 204-135-8
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
Retinaldehyde
CAS-No. 116-31-4 Acute Tox. 4; Skin Irrit. 2; <= 100 %
EC-No. 204-135-8 H302, H315
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
Retinaldehyde
CAS-No. 116-31-4 Xn, R22 - R38 <= 100 %
EC-No. 204-135-8
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Recommended storage temperature: -20 °C
Light sensitive.
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Full contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
Splash contact
Material: Nitrile rubber
Minimum layer thickness: 0,11 mm
Break through time: 480 min
Material tested:Dermatril® (KCL 740 / Z677272, Size M)
data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374
If used in solution, or mixed with other substances, and under conditions which differ from EN 374,
contact the supplier of the CE approved gloves. This recommendation is advisory only and must
be evaluated by an industrial hygienist and safety officer familiar with the specific situation of
anticipated use by our customers. It should not be construed as offering an approval for any
specific use scenario.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: powder
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 62 - 64 °C
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
mouse
lymphocyte
DNA inhibition
rat
mammary gland
DNA inhibition
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
Laboratory experiments have shown teratogenic effects.
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: VH6407000
Gastrointestinal disturbance, Repeated exposure may cause skin dryness or cracking., Headache
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
维生素A醛,又称视黄醛,是与A酸最接近的一种成分。它由β-胡萝卜素通过氧化断裂生成。还原后可得到视黄醇,进一步氧化则形成视黄酸。视黄醛作为视紫红质的辅基,在视觉细胞内,11-顺式视黄醛与视蛋白组成视色素。在光线的作用下,11-顺式视黄醛异构化为全反式视黄醛,导致视紫红质结构变化,从而引发大脑神经脉冲并形成视觉。
生理作用视黄醛是眼球发育过程中的重要信号转导分子,在脊椎动物的眼球发育中发挥多种关键作用。在网膜中,11-顺式视黄醛由全反式视黄醛或11-顺式视黄醇(新维生素Ab)通过酶促反应生成。视色素存在于视网膜感觉细胞中,是自然选择和进化过程中适应特定光环境产生的视觉物质,其实质是一种以生色团为辅基的色素蛋白。
保存条件纯度高的视黄醛固体对紫外光敏感且容易氧化,需要在避光并低温密封的条件下储存。
应用视黄醛作为一种护肤活性成分,具有抗皱、抗衰老和抗粉刺的功效。相较于其他常用的维生素A衍生物(如维A酸),它对皮肤的刺激性较小,特别适合敏感肌肤使用。经过人体代谢转化成视黄酸后,其还具备治疗粉刺的作用,是一种多功能性的活性添加剂。推荐在抗皱、抗衰老及抗粉刺护肤品配方中添加0.05%至0.15%浓度的视黄醛。
概述视网膜感觉细胞中的视色素含有视黄醛。食物中的维生素A和胡萝卜素经过肠道吸收并在体内转化为视黄醛,两者在视杆和视锥细胞内都存在,但由于结合蛋白的不同导致对光刺激的反应有所差异。视杆细胞中视黄醛通常以11—顺形式存在,在光照下转变为全反型视黄醛,进而引发视觉过程。暗处时,全反型视黄醛通过酶的作用重新转化为11—顺视黄醛,与视蛋白结合形成视紫红质,这一循环称为视循环。维生素A缺乏将导致视杆细胞光化学反应异常,引起夜盲症。
作用视黄醛作为眼球发育中的重要信号转导分子,在脊椎动物的眼球发育中发挥着多种关键作用。近视是发育性疾病,巩膜的主动扩张被认为是其增长的关键机制,而视黄醛可能成为调节实验性近视眼球增长的信息分子。研究显示了视黄醛及其核受体与实验性近视发生及发展之间的关系。
维A酸在皮肤科应用广泛,但由于局部刺激限制了它的临床使用范围。视黄醛作为天然维A酸的中间代谢产物,具有相似的生物活性,并且皮肤对它有更好的耐受性。本文综述了视黄醛及其生物学活性和在皮肤护理中的应用。
研究表明,对于戒烟20年后仍然存在患肺癌风险的人群来说,适量摄入视黄醛(即维生素A视黄醇和视黄酸衍生物)可以进一步降低这种可能性。得克萨斯大学M.D. Anderson癌症研究中心的Jonathan M. Kurie医学博士等人比较了两种不同类型的视黄醛对226名自愿者肺组织中RAR beta受体数量的影响,该受体减少通常被认为是发生前癌性肿瘤的先兆。经过9-cisRA三个月治疗后,发现自愿者的RAR beta受体数显著增加;而另一种视黄醛的效果则无明显差异。专家认为这一效应对吸烟人群可能有所不同。
化学性质存在16种立体异构体,其中13种已被化学合成,包括11-顺式为天然视蛋白中的发色物质。全E型晶体状,熔点64-65℃;7E、9Z、11E、13E型,熔点56-58℃。
用途作为β-胡萝卜素的中间体使用。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— retinal —— C20H28O 284.442 —— 7-cis-retinal 24315-14-8 C20H28O 284.442 —— 11,13-dicis-retinal 564-88-5 C20H28O 284.442 11-顺式视黄醛 (11Z)-retinal 564-87-4 C20H28O 284.442 —— 9-cis,13-cis-retinal 23790-80-9 C20H28O 284.442 —— 7,13-dicis-retinal 56085-54-2 C20H28O 284.442 —— 7,9-dicis-retinal 56085-53-1 C20H28O 284.442 —— 7-cis,9-cis,11-cis-retinal —— C20H28O 284.442 9-顺式视黄醛 9-cis vitamin A aldehyde 514-85-2 C20H28O 284.442 —— 9,11-dicis-retinal 67711-05-1 C20H28O 284.442 13-顺式视黄醇 13-cis-vitamin A aldehyde 472-86-6 C20H28O 284.442 —— 7-cis,11-cis-retinal 67737-35-3 C20H28O 284.442 (7E,9E)-beta-离子亚基乙醛 (2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal 3917-41-7 C15H22O 218.339 β-阿朴胡萝卜素醛 trans-β-apo-8'-carotenal 1107-26-2 C30H40O 416.647 —— (3E,5E,7E)-6-methyl-8-(2,6,6-trimethyl-1-cyclohexenyl)-3,5,7-octatriene-2-one 17974-57-1 C18H26O 258.404 β-胡萝卜素 beta-carotene 7235-40-7 C40H56 536.885 维A酸 all-trans-retinoic-acid 302-79-4 C20H28O2 300.441 11,12-二氢视黄醛 11,12-tetrahydroretinal 41889-27-4 C20H30O 286.458 维生素A RETINOL 68-26-8 C20H30O 286.458 —— 9-trans-13-trans-dimethyl-7-(1,1,5-trimethyl-5-cyclohexen-6-yl)-7,9,11,13-nonatetraene-15-nitrile 20638-88-4 C20H27N 281.441 —— all-cis-retinonitrile 20638-88-4 C20H27N 281.441 —— β-apo-8'-carotenol 6541-41-9 C30H42O 418.663 —— 3-methyl-5-chloro-1-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,3-pentadiene 55732-70-2 C15H23Cl 238.801 beta-离子亚基乙腈 (2E,4E)-3-methyl-5-(2,6,6-trimethyl-cyclohex-1-enyl)-penta-2,4-dienenitrile 5299-98-9 C15H21N 215.338 甲基(2E,4E,6E,8E)-3,7-二甲基-9-(2,6,6-三甲基-1-环己烯基)壬-2,4,6,8-四烯酸酯 Methyl retinoate 339-16-2 C21H30O2 314.468 —— 3-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-propenal 4951-40-0 C12H18O 178.274 —— [(1E,3E,5E)-4-methyl-6-(2,6,6-trimethylcyclohexen-1-yl)hexa-1,3,5-trienyl]boronic acid 1005452-43-6 C16H25BO2 260.184 beta-紫罗酮 (E)-β-ionone 79-77-6 C13H20O 192.301 (EZ)-β-紫罗兰酮 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one 14901-07-6 C13H20O 192.301 维生素 A 醋酸酯 Retinol acetate 127-47-9 C22H32O2 328.495 —— Butyl-[(2E,4E,6E,8Z)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraen-(E)-ylidene]-amine 36076-04-7 C24H37N 339.564 —— 7,8-dihydroretinal 75917-44-1 C20H30O 286.458 —— 3-(2,6,6-Trimethylcyclohex-1-enyl)prop-2-enenitrile 72214-33-6 C12H17N 175.274 —— ((1E,3E)-2-methyl-4-(2,6,6-trimethylcyclohex-1-en-1-yl)buta-1,3-dien-1-yl)boronic acid 550364-43-7 C14H23BO2 234.146 3-去氢视黄醛 3,4-didehydroretinal 472-87-7 C20H26O 282.426 β-紫罗兰醇 β-ionol 22029-76-1 C13H22O 194.317 —— retinylidene-1,3-cyclooctanedione 73685-26-4 C28H38O2 406.609 —— retinylidene-1,3-cycloheptanedione —— C27H36O2 392.582 —— retinylidene-1,3-cyclohexanedione —— C26H34O2 378.555 —— 9-cis-retro-γ-retinal 66901-09-5 C20H28O 284.442 —— N-methoxy-N,5-dimethyl-7-(2,6,6-trimethyl-1-cyclohexenyl)-2,4,6-heptanetrienamide 141207-99-0 C19H29NO2 303.445 —— all-trans-retinal propylene dithioacetal 142893-61-6 C23H34S2 374.655 —— 13-cis retinal propylene dithioacetal 146609-18-9 C23H34S2 374.655 2-[[(2E,4E,6E,8E)-3,7-二甲基-9-(2,6,6-三甲基-1-环己烯基)壬-2,4,6,8-四烯亚基]氨基]乙烷磺酸 Tauret 133867-05-7 C22H33NO3S 391.575 视黄基亚基二甲酮 all-trans-retinylidene dimedone 70424-15-6 C28H38O2 406.609 乙烯-beta-离子醇 (1E)-1-(2,6,6-trimethylcyclohex-1-enyl)-3-methyl-1,4-pentadien-3-ol 59057-30-6 C15H24O 220.355 —— tBDMS-retinol 118353-70-1 C26H44OSi 400.72 —— retinylidene-4,6-di-t-butyl-1,3-cyclohexanedione —— C34H50O2 490.77 —— (S)-6-Amino-2-[(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraen-(Z)-ylideneamino]-hexanoic acid 130443-70-8 C26H40N2O2 412.616 —— (trimethyl-2,6,6 cyclohexene-1 yl)-9 dimethoxy-1,1 dimethyl-3,7 hydroxy-7 nonatriene-3,5,8 111728-21-3 C22H36O3 348.526 —— 4-Methyl-6-[(1E,3E)-2-methyl-4-(2,6,6-trimethyl-cyclohex-1-enyl)-buta-1,3-dienyl]-5,6-dihydro-2H-pyran-2-ol 134962-99-5 C20H30O2 302.457 1-吡咯烷羧酸,3-[[(3-溴苯基)氨基]甲基]-,1,1-二甲基乙基酯 2,6,6-trimethylcyclohex-1-enecarbaldehyde 432-25-7 C10H16O 152.236 - 1
- 2
- 3
- 4
- 5
- 6
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 13-顺式视黄醇 13-cis-vitamin A aldehyde 472-86-6 C20H28O 284.442 9-顺式视黄醛 9-cis vitamin A aldehyde 514-85-2 C20H28O 284.442 11-顺式视黄醛 (11Z)-retinal 564-87-4 C20H28O 284.442 —— 9-cis,13-cis-retinal 23790-80-9 C20H28O 284.442 —— 7-cis-retinal 24315-14-8 C20H28O 284.442 —— 11,13-dicis-retinal 564-88-5 C20H28O 284.442 —— 9,11-dicis-retinal 67711-05-1 C20H28O 284.442 (2E,4E,6E,8E,10E,12E)-2,7,11-三甲基-13-(2,6,6-三甲基-1-环己烯-1-基)-2,4,6,8,10,12-十三六烯醛 12'-apo-β-caroten-12'-al 1638-05-7 C25H34O 350.544 beta-阿朴-14'-胡萝卜醛 β-apo-14'-carotenal 6985-27-9 C22H30O 310.48 —— all-trans-retinyl-methyl-ketone 67517-37-7 C21H30O 298.469 —— β-C23-Keton 67597-14-2 C23H32O 324.506 (9Z)-β--胡萝卜素 9-cis-β-carotene 13312-52-2 C40H56 536.885 —— β-carotene —— C21H30 282.469 β-胡萝卜素 beta-carotene 7235-40-7 C40H56 536.885 —— 9,13-dimethyl-7-(1,1,5-trimethyl-5-cyclohexen-6-yl)-7,9,11,13-octatetraene —— C19H28 256.431 —— 15-cis-β-carotene 19361-58-1 C40H56 536.885 —— (1E,3E)-3-methyl-1-[2,6,6-trimethylcyclohex-1-enyl]hexa-1,3,5-triene 43219-55-2 C16H24 216.367 —— (E)-2-(3-methylbuta-1,3-dienyl)-1,3,3-trimethyl-cyclohex-1-ene 22255-47-6 C14H22 190.329 —— Ro 11-0976 6722-00-5 C22H30O2 326.479 异维A酸 13-cis-retinoic acid 4759-48-2 C20H28O2 300.441 9-顺式维甲酸 9-cis-retinoic acid 5300-03-8 C20H28O2 300.441 (2Z,4E,6Z,8E)-3,7-二甲基-9-(2,6,6-三甲基-1-环己烯基)壬-2,4,6,8-四烯酸 9,13-di-cis-retinoic acid 5352-74-9 C20H28O2 300.441 维A酸 all-trans-retinoic-acid 302-79-4 C20H28O2 300.441 —— retinoic acid —— C20H28O2 300.441 —— (2E,4E,6E,8E,10E)-5,9-dimethyl-11-(2,6,6-trimethylcyclohex-1-en-1-yl)undeca-2,4,6,8,10-pentaene-1-ol 6985-26-8 C22H32O 312.495 11,12-二氢视黄醛 11,12-tetrahydroretinal 41889-27-4 C20H30O 286.458 —— 13,14-dihydroretinal 172302-35-1 C20H30O 286.458 维生素A RETINOL 68-26-8 C20H30O 286.458 (2E,4Z,6E,8E)-3,7-二甲基-9-(2,6,6-三甲基-1-环己烯基)壬-2,4,6,8-四烯-1-醇 11-cis-retinol 22737-96-8 C20H30O 286.458 13-顺式视黄醇 2-cis-Vitamin-A 2052-63-3 C20H30O 286.458 —— 3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nontetraen-1-ol 34218-73-0 C20H30O 286.458 —— 9-trans-13-trans-dimethyl-7-(1,1,5-trimethyl-5-cyclohexen-6-yl)-7,9,11,13-nonatetraene-15-nitrile 20638-88-4 C20H27N 281.441 —— retinylimine 23352-58-1 C20H29N 283.457 全-反式-13,14-二氢视黄醇 13,14-dihydro-all-trans retinol 115797-14-3 C20H32O 288.473 —— (2E,4E,6E,8E,10E,12E)-2,7,11-trimethyl-13-(2,6,6-trimethylcyclohex-1-en-1-yl)trideca-2,4,6,8,10,12-hexaenoic acid 1053-30-1 C25H34O2 366.544 —— Bishomoretinoic acid methyl ester 6980-77-4 C23H32O2 340.506 —— (2E,4E,6E,8E)-1,3,7-trimethyl-9-(2’,6’,6’-trimethylcyclohex-1’-en-1’-yl)nona-2,4,6,8-tetraen-1-ol 67517-38-8 C21H32O 300.484 甲基(2E,4E,6E,8E)-3,7-二甲基-9-(2,6,6-三甲基-1-环己烯基)壬-2,4,6,8-四烯酸酯 Methyl retinoate 339-16-2 C21H30O2 314.468 —— 4-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohexa-1,3-dienecarbaldehyde 1071780-12-5 C23H30O 322.491 —— Retinyliden-malonsaeure-dinitril 6980-75-2 C23H28N2 332.489 —— retinal methylimine Schiff base 51424-44-3 C21H31N 297.484 全反式视黄醛肟 all-trans-retinal oxime 67890-46-4 C20H29NO 299.456 —— Retinyliden-acetylaceton 6991-16-8 C25H34O2 366.544 —— all-trans-13,14-dihydroretinoic acid 98299-59-3 C20H30O2 302.457 (2Z,4E,6Z,8Z)-3,7-二甲基-9-(2,6,6-三甲基-1-环己烯基)壬-2,4,6,8-四烯酸乙酯 ethyl (all-E)-retinoate 3899-20-5 C22H32O2 328.495 —— 3,7,11-trimethyl-13t-(2,6,6-trimethyl-cyclohex-1-enyl)-trideca-2t,4t,6t,8t,10t,12-hexaenoic acid ethyl ester 96177-47-8 C27H38O2 394.598 维生素 A 醋酸酯 Retinol acetate 127-47-9 C22H32O2 328.495 —— retinal propylimine Schiff base 53633-90-2 C23H35N 325.538 —— all-E-retinoic acid 2-hydroxyethyl ester 65563-57-7 C22H32O3 344.494 —— 2-[[(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenylidene]amino]ethanol 28528-99-6 C22H33NO 327.51 (2E,4E,6E,8E)-N-丁基-3,7-二甲基-9-(2,6,6-三甲基-1-环己烯基)壬-2,4,6,8-四烯-1-亚胺 trans-retinylidene-n-butylamine 36076-04-7 C24H37N 339.564 —— Butyl-[(2E,4E,6E,8Z)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraen-(E)-ylidene]-amine 36076-04-7 C24H37N 339.564 —— all-E-retinoic acid 2-hydroxypropyl ester —— C23H34O3 358.521 —— 4-Hydroxybutyl all-trans-retinoate —— C24H36O3 372.548 —— 3-[(2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraen-(Z)-ylideneamino]-propionic acid 144459-74-5 C23H33NO2 355.521 —— Retinal-pinacol 18454-63-2 C40H58O2 570.899 —— retinylidene-1,3-cyclooctanedione 73685-26-4 C28H38O2 406.609 —— 2-[(1E,3E,5E,7E)-9,9-diethoxy-3,7-dimethylnona-1,3,5,7-tetraenyl]-1,3,3-trimethylcyclohexene 72612-82-9 C24H38O2 358.565 (2Z,4E,6Z,8E)-3,7-二甲基-9-(2,6,6-三甲基-3-氧代-1-环己烯基)壬-2,4,6,8-四烯醛 4-oxoretinal 33532-44-4 C20H26O2 298.425 —— 4-keto-9-cis-retinal —— C20H26O2 298.425 —— 4-oxo-13-cis-retinal 71423-69-3 C20H26O2 298.425 —— 4-keto-9,13-dicis-retinal 33532-44-4 C20H26O2 298.425 —— retinylidene-1,3-cycloheptanedione —— C27H36O2 392.582 —— retinylidene-1,3-cyclohexanedione —— C26H34O2 378.555 —— trans-retinal 1,3-dioxolane 105539-21-7 C22H32O2 328.495 —— 4-[[(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenylidene]amino]butanoic acid 94476-66-1 C24H35NO2 369.547 —— 6-[(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenylidene]azaniumylhexanoate —— C26H39NO2 397.6 4-酮维生素A all-trans-4-oxoretinol 62702-55-0 C20H28O2 300.441 —— (+/-)-4-Hydroxyretinal 18344-42-8 C20H28O2 300.441 —— 8-[(2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraen-(Z)-ylideneamino]-octanoic acid 144459-75-6 C28H43NO2 425.655 —— (2E,4E,6E,8E)-9-(3-Bromo-2,6,6-trimethyl-cyclohex-1-enyl)-3,7-dimethyl-nona-2,4,6,8-tetraenal 78508-49-3 C20H27BrO 363.338 2-[[(2E,4E,6E,8E)-3,7-二甲基-9-(2,6,6-三甲基-1-环己烯基)壬-2,4,6,8-四烯亚基]氨基]乙烷磺酸 Tauret 133867-05-7 C22H33NO3S 391.575 4-氧代-维A酸 4-ketoretinoic acid 38030-57-8 C20H26O3 314.425 —— 4-hydroxyretinoic acid 66592-72-1 C20H28O3 316.441 视黄基亚基二甲酮 all-trans-retinylidene dimedone 70424-15-6 C28H38O2 406.609 —— all-trans-4-oxo-DRA —— C20H28O3 316.441 —— all-trans-4-hydroxyretinol 15353-44-3 C20H30O2 302.457 —— all-trans-retinal dimer 193532-21-7 C40H54O 550.868 —— (3E,5E,7E,9E)-4,8-Dimethyl-10-(2,6,6-trimethyl-cyclohex-1-enyl)-2-trimethylsilanyloxy-deca-3,5,7,9-tetraenenitrile 292060-57-2 C24H37NOSi 383.649 —— all-trans-4-hydroxy-DRA —— C20H30O3 318.456 —— (3E,5E,7E,9E)-1-(4-Hydroxy-phenyl)-4,8-dimethyl-10-(2,6,6-trimethyl-cyclohex-1-enyl)-deca-3,5,7,9-tetraen-2-one —— C27H34O2 390.566 —— retinylidene-4,6-di-t-butyl-1,3-cyclohexanedione —— C34H50O2 490.77 维甲酰酚胺 N-(4-hydroxyphenyl)-all-trans-retinamide 65646-68-6 C26H33NO2 391.554 —— (S)-6-Amino-2-[(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraen-(Z)-ylideneamino]-hexanoic acid 130443-70-8 C26H40N2O2 412.616 —— all-trans-15-cyano-15'-pyridylretinal 1233340-02-7 C27H32N2 384.564 —— retinal tert-butyl-dimethylsilylcyanohydrin 886226-24-0 C27H43NOSi 425.73 —— 14-Hydroxy-retro-retinol —— C20H30O2 302.5 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
反应信息
-
作为反应物:参考文献:名称:9-顺式-类维生素A的催化合成:机理见解。摘要:热力学稳定的全反式类维生素A的区域选择性Z异构化仍然具有挑战性,并最终限制了治疗人类疾病急需的疗法的可用性。我们在这里提出了一种使用传统热处理或微波辐射催化类视黄醇 Z 异构化的新颖、简单的方法。通过筛选 20 种过渡金属催化剂,确定了区域选择性生产 Z-类维生素A 的最佳方法。最有效的催化系统由具有不稳定配体的钯配合物组成。多项机理研究,包括同位素 H/D 交换和使用耦合簇方法的最先进的量子化学计算表明,异构化是由催化剂二聚引发的,随后形成环状六元氯钯酸催化剂-底物加合物,最终打开生成所需的 Z 异构体。这里描述的合成开发,结合对基础化学的彻底机械分析,强调了以简单的形式使用现成的过渡金属催化剂来进行克级药物合成。DOI:10.1039/c9dt02189b
-
作为产物:参考文献:名称:A new approach to retinoids via organometallic addition to pyrylium salts摘要:描述了一种新的简洁方法,通过有机金属添加反应合成视黄素,该方法基于吡啶盐的反应;使用4-甲基吡啶四氟硼酸盐可以得到13Z-视黄素,该化合物可以很容易地异构化为视黄素本身;相应的未取代和4-环己基吡啶盐被转化为13-H和13-环己基视黄素类似物。DOI:10.1039/c39940002623
文献信息
-
[EN] (HETERO)ARYL CYCLOPROPYLAMINE COMPOUNDS AS LSD1 INHIBITORS<br/>[FR] COMPOSÉS D'(HÉTÉRO)ARYL-CYCLOPROPYLAMINE À TITRE D'INHIBITEURS DE LSD1申请人:ORYZON GENOMICS SA公开号:WO2013057322A1公开(公告)日:2013-04-25The invention relates to (hetero)aryl cyclopropylamine compounds, including particularly the compounds of formula (I) as described and defined herein, and their use in therapy, including, e.g., in the treatment or prevention of cancer, a neurological disease or condition, or a viral infection.本发明涉及(杂)芳基环丙胺化合物,特别是如本文所述和定义的公式(I)的化合物,以及它们在治疗中的应用,例如,在治疗或预防癌症、神经系统疾病或状况、或病毒感染方面的应用。
-
[EN] (HETERO)ARYL CYCLOPROPYLAMINE COMPOUNDS AS LSD1 INHIBITORS<br/>[FR] COMPOSÉS (HÉTÉRO)ARYLE CYCLOPROPYLAMINES EN TANT QU'INHIBITEURS DE LSD1申请人:ORYZON GENOMICS SA公开号:WO2013057320A1公开(公告)日:2013-04-25The invention relates to (hetero)aryl cyclopropylamine compounds, including particularly the compounds of formula I as described and defined herein, and their use in therapy, including, e.g., in the treatment or prevention of cancer, a neurological disease or condition, or a viral infection.本发明涉及(杂)芳基环丙胺化合物,特别是如本文所述和定义的公式I的化合物,以及它们在治疗中的用途,例如,在治疗或预防癌症、神经疾病或状况、或病毒感染中的用途。
-
[EN] SMALL MOLECULE ALDEHYDE DEHYDROGENASE INHIBITORS AND METHODS OF USE THEREOF<br/>[FR] PETITES MOLÉCULES INHIBITEURS DES ALDÉHYDE-DÉSHYDROGÉNASES ET LEURS MÉTHODES D'UTILISATION申请人:US HEALTH公开号:WO2016086008A1公开(公告)日:2016-06-02Described herein are compounds, salts and solvates of the formula (I). Certain compounds of formula (I) are potent and selective inhibitors of aldehyde dehydrogenases (ALDH), a family of enzymes that play a critical role in detoxification of various cytotoxic xenogenic and biogenic aldehydes. As such, compounds of formula (I) are useful for treating disorders in which ALDH inhibition is needed, including cancer, inflammatory disorders, and obesity. The disclosure also includes compositions, and methods for inhibiting aldehyde dehydrogenases (ALDH).
-
SULPHUR-LINKED COMPOUNDS FOR TREATING OPHTHALMIC DISEASES AND DISORDERS申请人:Scott Ian L.公开号:US20100093865A1公开(公告)日:2010-04-15Provided are sulphur-linked compounds, pharmaceutical compositions thereof, and methods of treating ophthalmic diseases and disorders, such as age-related macular degeneration and Stargardt's Disease, using said compounds and compositions.提供的是硫连接化合物、其药物组合物以及使用所述化合物和组合物治疗眼科疾病和障碍的方法,例如年龄相关的黄斑变性和斯达加特病。
-
[EN] OCTAHYDROCYCLOPENTAPYRROLES, THEIR PREPARATION AND USE<br/>[FR] OCTAHYDROCYCLOPENTAPYRROLES, LEUR PRÉPARATION ET LEUR UTILISATION申请人:UNIV COLUMBIA公开号:WO2014152018A1公开(公告)日:2014-09-25The present invention provides Octahydrocyclopentapyrrole compounds having the structure: (structurally represented) wherein psi is absent or present, and when present is a bond; R1, R2, R3, R4, and R5 are each independently H, halogen, CF, or C1-C4 alkyl; R6 is absent or present, and when present is H, OH, or halogen; A is absent or present, and when present is C(O) or C(O)NH; B is substituted or unsubstituted monocycle, bicycle, heteromonocycle, heterobicycle, benzyl, CO2H or (C1-C4 alkyl)-CO2H, wherein when B is CO2H, then A is present and is C(O); and when psi is present, then R6 is absent and when psi is absent, then R6 is present, or a pharmaceutically acceptable salt thereof, for treatement of diseases characterized by excessive lipofuscin accumulation in the retina.本发明提供了具有以下结构的八氢环戊吡咯化合物:(结构表示) 其中psi为不存在或存在,当存在时为键;R1、R2、R3、R4和R5各自独立为H、卤素、CF或C1-C4烷基;R6不存在或存在,当存在时为H、OH或卤素;A不存在或存在,当存在时为C(O)或C(O)NH;B为取代或未取代的单环、双环、杂单环、杂双环、苄基、CO2H或(C1-C4烷基)-CO2H,其中当B为CO2H时,A存在且为C(O);且当psi存在时,R6不存在,当psi不存在时,R6存在,或其药用可接受盐,用于治疗以视网膜过度脂褐素积聚为特征的疾病。
表征谱图
-
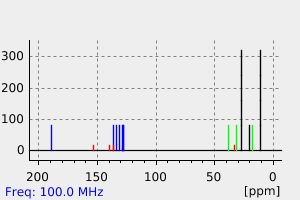
氢谱1HNMR
-
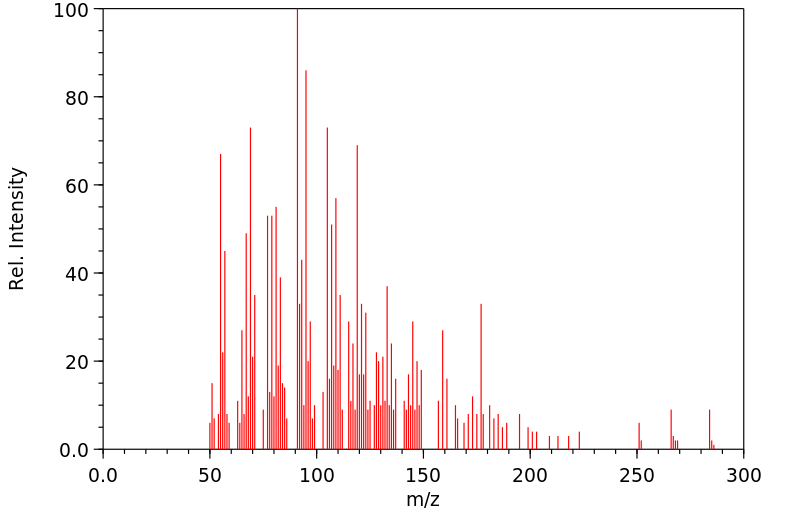
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息