甜橙提取物 | 5989-27-5
中文名称
甜橙提取物
中文别名
(+)-柠檬烯;右旋萜二烯;d-柠檬烯;D-柠檬烯;(R)-(+)-柠檬烯;(+)-柠檬烯((R)-(+)-苎烯)/D-柠檬烯;(R)-(+)-苎烯
英文名称
D-limonene
英文别名
(+)-Limonene;(R)-(+)-limonene;(4R)-limonene;(R)-1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene;α-limonene;(R)-(+)-4-isopropenyl-1-methyl-cyclohexene;(4R)-1-methyl-4-prop-1-en-2-ylcyclohexene;(R)-4-isopropenyl-1-methyl-1-cyclohexene;1-methyl-4-prop-1-en-2-ylcyclohexene;4-isopropenyl-1-methylcyclohexene;(4R)-1-methyl-4-(1-methylethenyl)cyclohexene;(+)-p-mentha-1,8-diene;(R)-p-mentha-1,8-diene;d‐limonene;(S)-limonene;Limonene, (+)-
CAS
5989-27-5;68647-72-3
化学式
C10H16
mdl
MFCD00062991
分子量
136.237
InChiKey
XMGQYMWWDOXHJM-JTQLQIEISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-75--73°C
-
比旋光度:112o (10% IN ETHANOL)
-
沸点:176-177 °C(lit.)
-
密度:0.842 g/mL at 20 °C(lit.)
-
蒸气密度:4.7 (vs air)
-
闪点:119 °F
-
溶解度:水:不溶
-
LogP:4.38 at 25℃
-
物理描述:D-limonene is a clear colorless mobile liquid with a pleasant lemon-like odor. (NTP, 1992)
-
颜色/状态:Oil
-
气味:Citrus odor
-
味道:FRESH, CITRUS TASTE
-
蒸汽密度:4.69 (NTP, 1992) (Relative to Air)
-
蒸汽压力:1.98 mm Hg at 25 °C
-
大气OH速率常数:1.71e-10 cm3/molecule*sec
-
稳定性/保质期:
Oxidizes to a film in air, oxidation behavior similar to that of rubber or drying oils. /Limonene/
-
旋光度:Specific optical rotation: +123.8 deg at 19.5 °C/D
-
自燃温度:237 °C
-
分解:When heated to decomp it emits acrid smoke and fumes.
-
燃烧热:19,250 Btu/lb = -10,840 cal/g = -454X10+5 J/kg
-
汽化热:140 Btu/lb
-
表面张力:26 dynes/cm
-
折光率:Index of refraction: 1.4730 at 20 °C/D
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
Incubation of d-limonene with rat liver microsomes produced d-limonene-1,2-diol and d-limonene-8,9-diol as metabolites and d-limonene-1,2-epoxide and d-limonene-8,9-epoxide were identified as intermediates.
来源:Hazardous Substances Data Bank (HSDB)
代谢
口服放射性碳标记的D-柠檬烯后,从狗和老鼠尿液中分离出5种新的代谢物:2-羟基-p-薄荷-8-烯-7-酸、香芹甘氨酸、香芹基-β-D-葡萄糖苷尿酸、p-薄荷-1,8-二烯-6-醇,以及可能是p-薄荷-1-烯-6,8,9-三醇。
After oral administration of (14)C-labeled d-limonene, 5 new metabolites were isolated from dog and rat urine: 2-hydroxy-p-menth-8-en-7-oic acid, perillylglycine, perillyl-beta-d-glucopyranosiduronic acid, p-mentha-1,8-dien-6-ol, and probably p-menth-1-ene-6,8,9-triol.
来源:Hazardous Substances Data Bank (HSDB)
代谢
尿液中的D-柠檬烯的主要代谢物在大鼠和兔子中是 perillic acid 8,9-diol,在仓鼠中是 perillyl-beta-d-glucopyranosiduronic acid,在狗中是 p-menth-1-ene-8,9-diol,在豚鼠和人中是 8-hydroxy-p-menth-1-ene-9-yl-beta-d-glucopyranosiduronic acid。
The major metabolite of d-limonene in urine was perillic acid 8,9-diol in rats and rabbits, perillyl-beta-d-glucopyranosiduronic acid in hamsters, p-menth-1-ene-8,9-diol in dogs, and 8-hydroxy-p-menth-1-ene-9-yl-beta-d-glucopyranosiduronic acid in guinea pigs and man.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Sprague Dawley 大鼠的成年雄性和雌性被给予了单一口服剂量的 0、0.1、0.3、1 或 3 毫摩尔 d-柠檬烯/千克(0、14、41、136 或 409 毫克/千克)的玉米油。凝胶过滤高效液相色谱(HPLC)表明,在雄性大鼠肾脏中的 d-柠檬烯与一种分子量约为 20000 的蛋白部分相关。使用反相高效液相色谱,显示 d-柠檬烯与 alpha-2u-球蛋白相关,后者通过氨基酸测序被鉴定。与 alpha-2u-球蛋白相关的主要代谢物是 d-柠檬烯-1,2-氧化物。母体 d-柠檬烯也被鉴定为 alpha-2u-球蛋白部分中的次要成分。
Adult male and female Sprague Dawley rats were given single oral doses of 0, 0.1, 0.3, 1, or 3 mmol d-limonene/kg (0, 14, 41, 136, or 409 mg/kg) in corn oil. Gel filtration HPLC indicated that d-limonene in male rat kidney is associated with a protein fraction having a mol wt of approximately 20000. Using reverse phase HPLC, d-limonene was shown to be associated with alpha-2u-globulin which was identified by amino acid sequencing. The major metabolite associated with alpha-2u-globulin was d-limonene-1,2-oxide. Parent d-limonene was also identified as a minor component in the alpha-2u-globulin fraction.
来源:Hazardous Substances Data Bank (HSDB)
代谢
(+)-limonene has known human metabolites that include Limonen-10-ol and Carveol.
来源:NORMAN Suspect List Exchange
毒理性
识别和使用:D-柠檬烯是一种无色液体或油,具有柑橘气味。D-柠檬烯是柑橘油的主要成分,是一种单萜烯,因其具有愉悦的类似柠檬的香气,被广泛用作化妆品、食品和工业溶剂中的香料/香气添加剂。它在美国注册为杀虫剂使用,但批准的杀虫剂用途可能会定期更改,因此必须咨询联邦、州和地方当局以获取当前批准的用途。D-柠檬烯还用于动物和人类的胆结石溶解。人类暴露和毒性:在多种消费品中广泛使用这种物质后,报告了皮肤刺激或致敏潜力。在人类中,D-柠檬烯的氧化产物或代谢物被证明是皮肤刺激物。皮肤刺激的潜在发生需要将这种化学物质作为化妆品成分进行规范。在人类中,D-柠檬烯在单次和重复剂量(长达一年)后表现出低毒性。报告称D-柠檬烯显著损害并增加了人类肺成纤维细胞膜的渗透性。动物研究:在实验动物中,D-柠檬烯的氧化产物或代谢物被证明是皮肤刺激物。根据口服给予动物的致死剂量(LD50)和重复剂量毒性研究,D-柠檬烯被指定为低毒性化学物质。在雄性大鼠中观察到致癌效应,但作用模式(MOA)被认为对人类无关,因为负责这种效应的啮齿动物蛋白α(2u)-球蛋白在人类中不存在。D-柠檬烯在雄性大鼠中诱导透明滴状肾病变。口服给予D-柠檬烯后,肝脏被确定为关键靶器官。从妊娠第7天至第12天连续6天给小鼠口服D-柠檬烯(2363 mg/kg)降低了体重增加并增加了胎儿异常骨形成的发病率。D-柠檬烯也降低了雄性后代的体重增加;然而,D-柠檬烯的毒性并不严重。D-柠檬烯在四种沙门氏菌菌株(TA 98、TA 100、TA 1535或TA 1537)中不具有诱变性,在鼠标L5178Y/TK +或-分析中未显著增加三氟胸腺嘧啶抗性细胞的数量,也未在培养的CHO细胞中诱导染色体畸变或姐妹染色单体交换。所有分析都是在存在和不存在外源代谢激活的情况下进行的。
IDENTIFICATION AND USE: D-Limonene is a colorless liquid or oil with citrus odor. D-Limonene, a major constituent of citrus oils, is a monoterpene widely used as a flavor/fragrance additive in cosmetics, foods, and industrial solvents as it possesses a pleasant lemon-like odor. It is registered for pesticide use in the USA but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. D-Limonene is also used as gallstone solubilizer in animals and humans. HUMAN EXPOSURE AND TOXICITY: Skin irritation or sensitizing potential was reported following widespread use of this agent in various consumer products. In humans, oxidation products or metabolites of d-limonene were shown to act as skin irritants. The potential occurrence of skin irritation necessitates regulation of this chemical as an ingredient in cosmetics. In humans, d-limonene has demonstrated low toxicity after single and repeated dosing for up to one year. D-Limonene was reported to significantly damage and increase permeability of membranes of human lung fibroblasts. ANIMAL STUDIES: In experimental animals oxidation products or metabolites of d-limonene were shown to act as skin irritants. D-Limonene has been designated as a chemical with low toxicity based upon lethal dose (LD50) and repeated-dose toxicity studies when administered orally to animals. Carcinogenic effects have also been observed in male rats, but the mode of action (MOA) is considered irrelevant for humans as the protein alpha(2u)-globulin responsible for this effect in rodents is absent in humans. d-Limonene induced hyaline droplet nephropathy in male rats. The liver was identified as a critical target organ following oral administration of d-limonene. D-Limonene (2363 mg/kg, orally) given to mice for 6 days from day 7-12 of gestation decreased body weight gain and increased incidence of abnormal bone formation in fetuses. D-Limonene also decreased body weight gain in male offspring; however, the toxicity of d-limonene was not severe. D-Limonene was not mutagenic in four strains of Salmonella typhimurium (TA 98, TA 100, TA 1535, or TA 1537), did not significantly increase the number of trifluorothymidine-resistant cells in the mouse L5178Y/TK + or - assay, and did not induce chromosomal aberrations or sister chromatid exchanges in cultured CHO cells. All assays were conducted in the presence and absence of exogenous metabolic activation.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:对于d-柠檬烯对人类致癌性的证据不足。对于d-柠檬烯对实验动物致癌性的证据是充分的。总体评估:在对其人类致癌性的总体评估中,工作组认为d-柠檬烯通过一种与非DNA反应的α-2u-球蛋白相关的反应,在雄性大鼠中产生肾小管肿瘤。因此,d-柠檬烯增加雄性大鼠肾小管肿瘤发生率的机制对人类不相关。d-柠檬烯对其人类致癌性无法分类(第3组)。
Evaluation: There is inadequate evidence in humans for the carcinogenicity of d-limonene. There is sufficient evidence in experimental animals for the carcinogenicity of d-limonene. Overall evaluation: In making its overall evaluation of the carcinogenicity to humans of d-limonene, the Working Group concluded that d-limonene produces renal tubular tumors in male rats by a non-DNA reactive alpha-2u-globulin associated response. Therefore, the mechanism by which d-limonene incr the incidence of renal tubular tumors in male rats is not relevant to humans. d-Limonene is not classifiable as to its carcinogenicity to humans (Group 3).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:d-柠檬烯
IARC Carcinogenic Agent:d-Limonene
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:对其对人类的致癌性无法分类
IARC Carcinogenic Classes:Group 3: Not classifiable as to its carcinogenicity to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构专著:第56卷:(1993年)一些自然存在的物质:食品成分和构成要素,杂环芳香胺和霉菌毒素
IARC Monographs:Volume 56: (1993) Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
在人和长埃文斯大鼠中评估了外用[(14)C]d-柠檬烯的命运。在大鼠中,将5 mg/kg体重的[(14)C]d-柠檬烯外用涂抹在剃光的背部并封闭,大约有12%的剂量被吸收。吸收的d-柠檬烯被完全代谢并迅速排出,主要从尿液中排出(80%),小部分(20%)通过粪便排出。测试材料在体内组织中没有长期滞留。在人类中,将12 mg的[(14)C]d-柠檬烯溶于乙醇(1 mL)外用在背部,在非封闭条件下涂抹1小时后让物质干燥,然后封闭,只有0.16%的剂量被吸收,放射性物质从尿液中回收。人类粪便中的放射性物质低于检测限。这些结果表明,在模拟使用香水和化妆品的条件下,d-柠檬烯的皮肤吸收和积聚潜力较低,且吸收的d-柠檬烯会迅速通过尿液排出。基于这些发现以及d-柠檬烯具有低系统性毒性的知识,可以合理地得出结论,从香水和化妆品应用中经皮暴露于d-柠檬烯极不可能导致任何具有临床意义的人类毒性。
The fate of dermally applied [(14)C]d-limonene was evaluated in humans and Long-Evans rats. In rats, 5 mg/kg body weight of [(14)C]d-limonene applied dermally to the shaved back under occlusion, resulted in the absorption of approximately 12% of the dose. The absorbed d-limonene was completely metabolized and excreted rapidly, primarily from the urine (80%) with a small fraction (20%) excreted in the feces. There was no long-term retention of the test material in body tissues. In humans, following dermal application of 12 mg of [(14)C]d-limonene in ethanol (1 mL) to the back under nonocclusive conditions (for 1 h after application to allow the material to dry, thereafter under occlusion), only 0.16% of the dose was absorbed and the radioactivity was recovered from the urine. Radioactivity in human feces was below the limit of detection. These results indicate that under conditions of simulated use of fragrances and cosmetics, d-limonene has a low potential for dermal absorption and tissue accumulation, and the d-limonene that is absorbed is rapidly excreted in the urine. Based upon these findings and the knowledge that d-limonene possesses a low-systemic toxicity profile, it is reasonable to conclude that dermal exposure to d-limonene from fragrance and cosmetic applications is highly unlikely to result in any clinically significant human toxicity.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
口服标记有(14)C的d-柠檬烯后,2到3天内,动物和人体分别有75-95%和小于10%的放射性物质通过尿液和粪便排出。
After oral administration of (14)C-labeled d-limonene to animals and humans, 75-95 and <10% of the radioactivity was excreted in the urine and feces respectively within 2-3 days.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
d-柠檬烯的毒物动力学在吸入暴露(2小时,工作负荷50瓦)的人体志愿者中进行研究,他们在不同的三次场合暴露于一个暴露室中。暴露浓度大约为10、225和450毫克/立方米d-柠檬烯。相对肺吸收很高,大约为供应量的70%。在这项研究中观察到的d-柠檬烯的血清除率为1.1升/千克/小时,这表明d-柠檬烯容易被代谢。在暴露结束后,大约1%的总吸收量未变地通过呼出的空气排出,而大约0.003%通过尿液排出。在缓慢排泄阶段观察到血液中的半衰期较长,这表明在脂肪组织中积累。
The toxicokinetics of d-limonene were studied in human volunteers exposed by inhalation (2 hr, work load 50 W) in an exposure chamber on three different occasions. The exposure concentrations were approximately 10, 225, and 450 mg/cu m d-limonene. The relative pulmonary uptake was high, approximately 70% of the amount supplied. The blood clearance of d-limonene observed in this study, 1.1 L/kg/hr, indicates that d-limonene is metabolized readily. About 1% of the total uptake was eliminated unchanged in the expired air after the end of exposure, while approximately 0.003% was eliminated in the urine. A long half-time in blood was observed in the slow elimination phase, which indicates accumulation in adipose tissues.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Orally administered d-limonene is rapidly and almost completely taken up from the gastrointestinal tract in humans as well as in animals. Infusion of labelled d-limonene into the common bile duct of volunteers revealed that the chemical was very poorly absorbed from the biliary system.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:Xn,Xi,N
-
安全说明:S24,S37,S60,S61
-
危险类别码:R38,R43,R10,R50/53,R65
-
WGK Germany:2,3
-
海关编码:2902199012
-
危险品运输编号:UN 2052 3
-
RTECS号:GW6360000
-
包装等级:III
-
危险标志:GHS02,GHS07,GHS08,GHS09
-
危险性描述:H226,H304,H315,H317,H410
-
危险性防范说明:P273,P280,P301 + P310,P331,P501
SDS
制备方法与用途
含量分析
按GT-10标准,使用极性柱进行气液色谱法分析。具体条件如下:不锈钢柱长300厘米×3厘米;柱填料基料为聚乙二醇20M,10%重量的固体载体;硅藻土经过热碱处理后硅化并酸洗,粒度为10~20目,平均自由落积密度为0.2克/立方厘米,粒径80~100目。载气采用氦气,流量30毫升/分钟;检测器为热导池;柱温范围在100~225℃之间,升温速率为4℃/分钟。进样量为0.2微升,通过峰面积计算含量。
毒性该物质被列为GRAS(美国食品和药物管理局FEMA及FAO/WHO 1994年标准),ADI无特殊规定。大鼠经口LD50值为4400毫克/千克。
使用限量- 软饮料:31毫克/千克
- 冷饮:68毫克/千克
- 糖果:49毫克/千克
- 焙烤食品:120毫克/千克
- 布丁类:48~400毫克/千克
- 胶姆糖:2300毫克/千克
无色油状液体,具有愉快新鲜橙子香气,不应带有樟脑和萜类气味。在空气和潮气的影响下会自行氧化成香芹油萜酮和香芹油萜醇,从而导致变质。沸点为177℃,闪点46℃。该物质混溶于乙醇及大多数非挥发性油中;微溶于甘油,不溶于水和丙二醇。
天然品存在于300多种精油中,特别是柑橘类精油如橙油(约90%)、柠檬油、橘油、白柠檬油、柚油、青柠檬油、橙花油、橙叶油、榄香脂、香芹子油(约40%)、莳萝油、小茴香油和芹子油(约60%)等中。
用途根据GB 2760—1996标准,该物质作为允许使用的食用香料。主要用于配制白柠檬、柑橘类及香辛料类香精。
生产方法从含有d-芋烯的天然精油(如橙油)中分馏出初品;加入金属钠重蒸一次,得到粗品;经溴化生成四溴芋烯,再在含锌粉的乙醇液中还原而得纯品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 双戊烯 d-limonene 138-86-3 C10H16 136.237 (S)-(-)-柠檬烯 (-)-(S)-limonene 5989-54-8 C10H16 136.237 (S)-(-)-紫苏醇 (-)-Perillyl alcohol 18457-55-1 C10H16O 152.236 —— p-menth-1-ene 27966-26-3 C10H18 138.253 (R)-香芹醇 L-carveol 308363-12-4 C10H16O 152.236 (1R,5S)-香芹醇 (+)-trans-carveol 18383-51-2 C10H16O 152.236 —— (R)-Carvone 2244-16-8 C10H14O 150.221 香芹酮 Carvone 99-49-0 C10H14O 150.221 —— (E)-2-[(2R,5R)-2-methyl-5-(prop-1-en-2-yl)cyclohexylidene]ethanol —— C12H20O 180.29 —— (2R)-2-[(1R)-4-methylcyclohex-3-en-1-yl]propan-1-ol 13835-75-1 C10H18O 154.252 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 双戊烯 d-limonene 138-86-3 C10H16 136.237 (R)-Β-异丁烯二烯 (+)-β-bisabolene 20377-48-4 C15H24 204.356 —— (4R)-9-heptyl-p-mentha-1,8(10)-diene 106026-45-3 C17H30 234.425 (S)-1-甲基-4-(5-甲基-1-亚甲基-4-己烯基)环己烯 (S)-1-methyl-4-(6-methylhepta-1,5-dien-2-yl)cyclohex-1-ene 495-61-4 C15H24 204.356 (1E,5E,9E,12R)-1,5,9-三甲基-12-丙-1-烯-2-基环十四烷并-1,5,9-三烯 (–)-(R)-cembrene A 31570-39-5 C20H32 272.474 —— (4R)-1-methyl-4-[(4'E)-5',9'-dimethyl-1'-methylene-4',8'-decadienyl]cyclohexene —— C20H32 272.474 —— (+)-R-E-α-bisabolene 70286-31-6 C15H24 204.356 —— (-)-(Z)-α-bisabolene 70286-33-8 C15H24 204.356 —— (1'R)-(2E)-3-methyl-3-(4'-methylcyclohexen-3'-yl)-propen-2-al-1 73088-68-3 C11H16O 164.247 —— (1'R)-(2Z)-3-methyl-3-(4'-methylcyclohexene-3'-yl)-propen-2-al-1 77647-35-9 C11H16O 164.247 —— 9-hydroxymethyl-1,8(10)-p-menthadiene 77614-30-3 C11H18O 166.263 —— (R)-3-(4-methyl-cyclohex-3-enyl)-but-3-enal 77614-31-4 C11H16O 164.247 2-(4-甲基-1-环己-3-烯基)丙-2-烯-1-醇 (R)-9-hydroxy-p-mentha-1,8(10)-diene 38142-45-9 C10H16O 152.236 柠檬烯-10-醇 Limonen-10-ol 3269-90-7 C10H16O 152.236 —— (3Z,6R)-3-methyl-6-(prop-1’-en-2’-yl)-dec-9-en-1-ol 72257-34-2 C14H24O 208.344 (S)-(-)-紫苏醇 (-)-Perillyl alcohol 18457-55-1 C10H16O 152.236 紫苏子醇 (R)-Perillyl alcohol 57717-97-2 C10H16O 152.236 紫苏醇 perillol 536-59-4 C10H16O 152.236 —— limonen-10-al 121962-40-1 C10H14O 150.221 —— 4-methyl-α-methylene-3-cyclohexene-1-acetaldehyde 57074-31-4 C10H14O 150.221 —— 4R-(+)-9-chloro-1,8-p-menthadiene —— C10H15Cl 170.682 (+)-紫苏醛 L-perillaldehyde 5503-12-8 C10H14O 150.221 (S)-(-)-紫苏醛 (-)-perillaldehyde 18031-40-8 C10H14O 150.221 (5Z)-2-甲基-6-(4-甲基-3-环己烯-1-基)-2,5-庚二烯-4-酮 (E)-α-atlantone 26294-59-7 C15H22O 218.339 —— trans α-atlantone 532-64-9 C15H22O 218.339 —— p-menth-1-ene 27966-26-3 C10H18 138.253 (+)-对-薄荷-1-烯 (+)-p-menth-1-ene 1195-31-9 C10H18 138.253 (4S)-异丙基-1-甲基环己烯 (S)-carvomenthene 499-94-5 C10H18 138.253 —— β-atlantone 38331-79-2 C15H22O 218.339 —— (3R,6E,10E)-6,10-dimethyl-3-isopropenylpentadeca-6,10-dien-1-al 79859-58-8 C20H32O2 304.473 —— β-atlantol 420109-31-5 C15H24O 220.355 —— p-mentha-1,8-dien-2-ol —— C10H16O 152.236 4-叔丁基-1-甲基环己烯 4-tert-Butyl-1-methylcyclohex-1-en 3419-74-7 C11H20 152.28 (-)-香芹酚 cis-2-methyl-5-(1-methylethenyl)-2-cyclohexen-1-ol 1197-06-4 C10H16O 152.236 —— (-)-trans-carveol 18383-51-2 C10H16O 152.236 —— carveol 188635-29-2 C10H16O 152.236 香芹醇 5-Isopropenyl-2-methyl-cyclohex-2-enol 99-48-9 C10H16O 152.236 (1R,5S)-香芹醇 (+)-trans-carveol 18383-51-2 C10H16O 152.236 (R)-香芹醇 L-carveol 308363-12-4 C10H16O 152.236 (2S,4S)-香芹醇 (2S,4S)-carveol 7632-16-8 C10H16O 152.236 对薄荷-3-烯 3-p-menthene 500-00-5 C10H18 138.253 —— (R)-Carvone 2244-16-8 C10H14O 150.221 香芹酮 Carvone 99-49-0 C10H14O 150.221 右旋香芹酮 (S)-p-mentha-6,8-dien-2-one 2244-16-8 C10H14O 150.221 —— (3Z,6R)-3-methyl-6-(prop-1’-en-2’-yl)-dec-9-en-1-yl acetate 67225-47-2 C16H26O2 250.381 —— 2,5-dimethyl-bicyclo[3.2.1]oct-2-ene 42370-20-7 C10H16 136.237 —— (R,E)-methyl 3-(4-methyl-3-cyclohexenyl)-2-butenoate 38142-44-8 C12H18O2 194.274 —— isopiperitenol —— C10H16O 152.236 —— 4-methyl-1-isopropenylcyclohex-3-en-1-ol 3419-02-1 C10H16O 152.236 —— isopiperitenol 65733-30-4 C10H16O 152.236 —— (-)-1,8-p-Menthadien-4-ol 38630-70-5 C10H16O 152.236 —— (1S,6S)-3-methyl-6-(prop-1-en-2-yl)cyclohex-2-enol 65733-29-1 C10H16O 152.236 —— (+)-(3S)-3-[(1R)-4-methyl-3-cyclohexen-1-yl]-1-butanol 262290-92-6 C11H20O 168.279 甲基环己烯基丁醇 3-(4-methyl-3-cyclohexen-1-yl)-1-butanol 15760-18-6 C11H20O 168.279 3-甲基-6-(1-甲基乙烯基)-2-环己烯-1-酮 (S)-isopiperitenone 16750-82-6 C10H14O 150.221 异辣薄荷烯酮 isopiperitenone 529-01-1 C10H14O 150.221 新柠檬醛; 3-(4-甲基-3-环己烯基)丁醛; beta,4-二甲基-3-环己烯-1-丙醛 1-Methyl-4-(2-butanalyl)-1-cyclohexene 6784-13-0 C11H18O 166.263 —— (R)-3-((R)-4-methylcyclohex-3-en-1-yl)butanal 128300-28-7 C11H18O 166.263 —— (S)-3-((R)-4-methylcyclohex-3-en-1-yl)butanal 128300-30-1 C11H18O 166.263 —— 3-(4-methylcyclohex-3-enyl)butanal 199445-85-7 C11H18O 166.263 —— (S)-3-((R)-4-methylcyclohex-3-en-1-yl)butanenitrile 26462-76-0 C11H17N 163.263 —— (3S)-3-[(1S)-4-Methylcyclohex-3-enyl]butanal 138117-27-8 C11H18O 166.263 —— (+)-(R)-3-(4-Methyl-3-cyclohexen-1-yl)-3-butensaeuremethylester 38142-43-7 C12H18O2 194.274 1-亚甲基-4-(1-甲基乙烯基)环己烷 pseudolimonene 499-97-8 C10H16 136.237 —— (2R)-2-[(1R)-4-methylcyclohex-3-en-1-yl]propan-1-ol 13835-75-1 C10H18O 154.252 2-(4-甲基-3-环己烯-1-基)丙醛 α-4-dimethyl-3-cyclohexene-1-acetaldehyde 29548-14-9 C10H16O 152.236 —— 2-[(1S)-4-methylcyclohex-3-en-1-yl]propanal 205926-46-1 C10H16O 152.236 —— 1-methyl-4-(1-methylcyclopropyl)-1-cyclohexene 60059-22-5 C11H18 150.264 —— Perilla-alkohol-acetat 56452-54-1 C12H18O2 194.274 —— 1,1,1-trichloro-4-(4-methylcyclohex-3-enyl)pent-4-en-2-ol 90454-95-8 C12H17Cl3O 283.625 —— (+)-β-phellandrene 6153-16-8 C10H16 136.237 3-亚甲基-6-丙-2-基-环己烯 (-)-β-phellandrene 6153-17-9 C10H16 136.237 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
反应信息
-
作为反应物:描述:参考文献:名称:Henry; Paget, Journal of the Chemical Society, 1928, p. 77摘要:DOI:
-
作为产物:参考文献:名称:为C-C的新方法末端烯烃的耦合通过一个磺酰烷基化-desulphinylation序列:合成ë -和ž -α-bisabolenes摘要:描述了一种新的方法,该方法基于烯丙基亚磺酸介导的基于磺酰化-烷基化-脱磺酰化过程的末端烯烃的区域特异性CC偶联及其在柠檬烯转化为E-和Z -α-双双硼烷中的应用。DOI:10.1039/c39880000702
-
作为试剂:参考文献:名称:A Scalable Synthesis of
l -Leucine-N-carboxyanhydride摘要:Due to its relevance in the synthesis of well-defined oligopeptides, the L-leucine-N-carboxyanhydride (leucine-NCA) synthesis was selected for fine chemical scale-up with a scope on application on larger scales. The heterogeneous gas-solid-liquid nature of the leucine-NCA synthesis implied a mass transfer limited reaction rate towards phosgenation and was investigated on bench scale. Upon scale increase, the liquid-gas mass transport of HCl is drastically reduced, retarding the reaction and consequently rendering the process unsuitable for scale-up. Addition of an HCl scavenger such as (+)-limonene prevented side reactions thus allowing a cost reduction, a considerably faster reaction, and minimization of the amount of phosgene source used. The modified leucine-NCA synthesis has successfully been made scalable, maintaining high product purity on a 1.0 dm(3) scale.DOI:10.1021/op058009e
文献信息
-
Ambient Hydrogenation and Deuteration of Alkenes Using a Nanostructured Ni‐Core–Shell Catalyst作者:Jie Gao、Rui Ma、Lu Feng、Yuefeng Liu、Ralf Jackstell、Rajenahally V. Jagadeesh、Matthias BellerDOI:10.1002/anie.202105492日期:2021.8.16selective hydrogenation and deuteration of a variety of alkenes is presented. Key to success for these reactions is the use of a specific nickel-graphitic shell-based core–shell-structured catalyst, which is conveniently prepared by impregnation and subsequent calcination of nickel nitrate on carbon at 450 °C under argon. Applying this nanostructured catalyst, both terminal and internal alkenes, which
-
Acidic functionalized ionic liquids as catalyst for the isomerization of α-pinene to camphene作者:Yue Liu、Lu Li、Cong Xia XieDOI:10.1007/s11164-015-2041-2日期:2016.2the selectivity for camphene could reach 64.8 %. In addition, the catalyst could be easily separated by centrifugation after the isomerization completely finished. When the ILs were repeatedly used for four times, the conversion of α-pinene and the selectivity for camphene were still excellent, indicating the superb recycle ability of the acidic functionalized ILs catalyst.合成了酸性功能化离子液体(ILs)[HSO 3-(CH 2)3 -NEt 3 ] Cl-ZnCl 2,并用于在均匀体系中催化α- pine烯的异构化。异构化的最佳条件如下:n(α-pine烯):n(ILs)= 9:1,反应温度140°C,反应时间4 h,α-0.0烯0.04 mol。在最佳条件下,α-pine烯的转化率为97.6%,对camp烯的选择性可达64.8%。另外,在异构化完全完成之后,可以通过离心容易地分离催化剂。当IL重复使用四次时,α-pine烯的转化率和对camp烯的选择性仍然非常好,表明酸性功能化ILs催化剂具有极好的循环能力。
-
Planar‐Chiral [2.2]Paracyclophane‐Based Pyridonates as Ligands for Tantalum‐Catalyzed Hydroaminoalkylation作者:Carolin Braun、Martin Nieger、Stefan Bräse、Laurel L. SchaferDOI:10.1002/cctc.201900416日期:2019.11.7By using planar chiral [2.2]paracyclophane‐containing N,O‐chelating ligands for tantalum‐catalyzed hydroaminoalkylation, one of the most versatile catalytic systems for this reaction to date was obtained. Convenient Csp3−Csp3 bond formation of amines with terminal and internal alkenes was enabled by the same in situ synthesized catalytic system of [2.2]paracyclophane‐based pyridonates and Ta(CH2TMS)3Cl2
-
Visible photocatalysis of novel oxime phosphonates: synthesis of β-aminophosphonates作者:Yong-Hong Li、Chun-Hai Wang、Su-Qian Gao、Feng-Ming Qi、Shang-Dong YangDOI:10.1039/c9cc06075h日期:——A novel type of oxime phosphonate was synthesized and used in the intermolecular cascade radical addition reaction of alkenes to access β-aminophosphonates via visible-light-driven N-centered iminyl radical-mediated and redox-neutral selective C–P single-bond cleavage in an active phosphorus radical route. The procedure is characterized by its ability to achieve the construction of Csp3–P and Csp3–N
-
Iron-Catalyzed Hydroboration: Unlocking Reactivity through Ligand Modulation作者:Maialen Espinal-Viguri、Callum R. Woof、Ruth L. WebsterDOI:10.1002/chem.201602818日期:2016.8.8hydroboration (HB) of alkenes and alkynes is reported. A simple change in ligand structure leads to an extensive change in catalyst activity. Reactions proceed efficiently over a wide range of challenging substrates including activated, unactivated and sterically encumbered motifs. Conditions are mild and do not require the use of reducing agents or other additives. Large excesses of borating reagent are not required
表征谱图
-
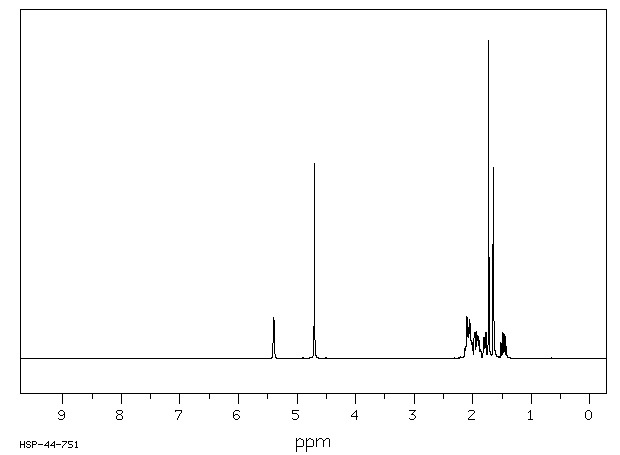
氢谱1HNMR
-
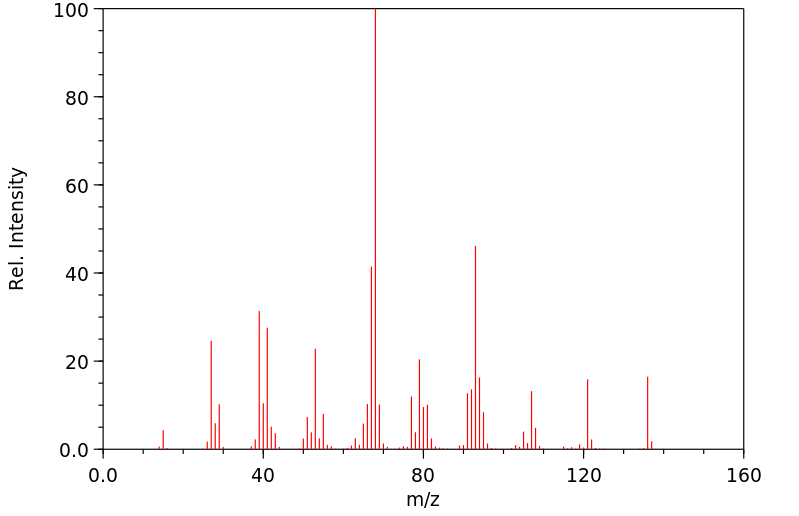
质谱MS
-
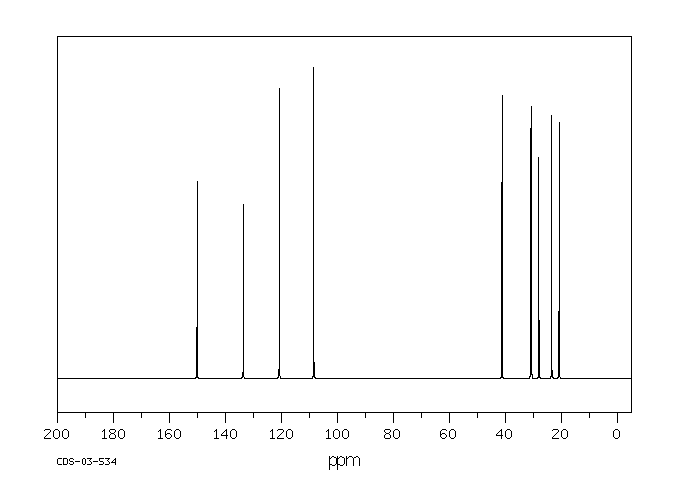
碳谱13CNMR
-
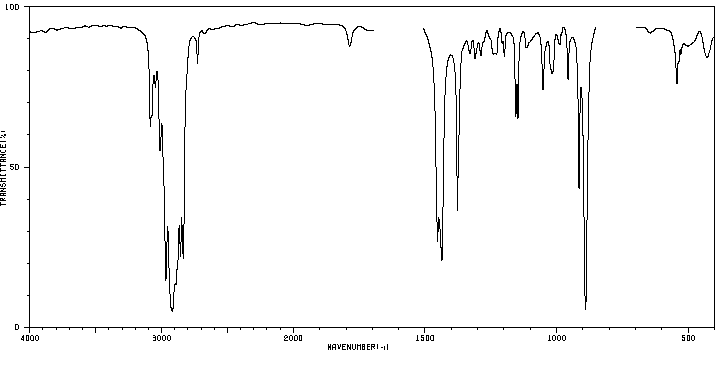
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸