1-亚甲基-4-(1-甲基乙烯基)环己烷 | 499-97-8
中文名称
1-亚甲基-4-(1-甲基乙烯基)环己烷
中文别名
假性柠檬烯
英文名称
pseudolimonene
英文别名
p-mentha-1(7),8-diene;1-methylene-4-(1-methylethenyl)cyclohexane;ψ-limonene;1-isopropenyl-4-methylenecyclohexane;mentha-1(7),8-diene;1-Methylene-4-(1-methylvinyl)cyclohexane;1-methylidene-4-prop-1-en-2-ylcyclohexane
CAS
499-97-8
化学式
C10H16
mdl
——
分子量
136.237
InChiKey
GOQRXDTWKVYHJH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:65-66 °C(Press: 11 Torr)
-
密度:0.8735 g/cm3
-
LogP:4.481 (est)
-
保留指数:992;993;990;1018
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:1-亚甲基-4-(1-甲基乙烯基)环己烷 在 溴 作用下, 以 乙醚 为溶剂, 反应 0.75h, 以0.12 g的产率得到(1RS,2SR,4RS,8RS)-1,2,7,8,9-pentabromo-p-menthane参考文献:名称:Halogenated Terpenoids. XXXI. Tribromides from the Bromination of Various Exocyclic Olefins摘要:环己基系统外环的亚甲基基团的溴化作用除了可以得到预期的反式二溴产物外,还可以得到相当数量的三溴化物。例如,简单溴化4-叔丁基-1-亚甲基环己烷可以得到约20%的产率,得到(r-1, t-2, c-4)-1,2-二溴-1-溴甲基-4-叔丁基环己烷。DOI:10.1071/ch00041
-
作为产物:描述:参考文献:名称:烯丙基醇通过甲锡烷基化/去甲锡烷基化反应脱氧为末端烯烃摘要:烯丙基醇的脱氧反应通过三个步骤进行;1)O-烯丙基黄原酸酯的[3,3]-σ重排,2)用氢化三丁基锡进行连续锡锡酸酯化,生成烯丙基锡烷,和3)最终将烯丙基锡烷酮分解为末端烯烃。DOI:10.1016/s0040-4039(00)77832-4
文献信息
-
Unique catalysis of gold nanoparticles in the chemoselective hydrogenolysis with H2: cooperative effect between small gold nanoparticles and a basic support作者:Akifumi Noujima、Takato Mitsudome、Tomoo Mizugaki、Koichiro Jitsukawa、Kiyotomi KanedaDOI:10.1039/c2cc32850j日期:——Gold nanoparticles on hydrotalcite act as a heterogeneous catalyst for the chemoselective hydrogenolysis of various allylic carbonates to the corresponding terminal alkenes using H2 as a clean reductant. The combination of gold nanoparticles and basic supports elicited significantly unique and selective catalysis in the hydrogenolysis.
-
[EN] PROCESS FOR PREPARING JET FUEL FROM MOLECULES DERIVED FROM BIOMASS<br/>[FR] PROCÉDÉ DE PRÉPARATION D'UN CARBURANT AVIATION À PARTIR DE MOLÉCULES DÉRIVÉES D'UNE BIOMASSE申请人:TOTAL RAFFINAGE MARKETING公开号:WO2013053740A1公开(公告)日:2013-04-18The invention relates to a process for preparing jet fuel or jet fuel precursors which comprises the treatment of a charge derived from biomass, the said charge comprising at least one compound chosen from terpenes of formula [CH2=C(CH3)CH=CH2]n, in which n is an integer of from 2 to 12, the carbon chain of which is linear, cyclic or branched, or cyclic or branched terpenes as defined previously, which have been chemically modified by oxidation and/or rearrangement of the carbon backbone, the said process comprising a cycloaddition step (i) followed by a cracking and hydrogenation step (ii).
-
Chemistry of organosilicon compounds—165作者:Hideki sakurai、Akira Hosomi、Masaki Saito、Koshi Sasaki、Hirokazu Iguchi、Jun-Ichi Sasaki、Yoshitaka ArakiDOI:10.1016/s0040-4020(01)88587-2日期:1983.1of synthetically useful reactions of 2-trimethylsilylmethyl-1,3-butadiene (7) are discussed. Reactions of 7 with acid chlorides, aldehydes, ketones and acetals activated by a Lewis acid give isoprenylated compounds, while 7 undergoes the Diels-Alder reaction with dienophiles. High regiospecificity of the reaction qualifies 7 for a versatile building block of terpene synthesis.
-
An efficient method for the reductive transposition of allylic alcohols作者:Andrew G Myers、Bin ZhengDOI:10.1016/0040-4039(96)00965-3日期:1996.7Mitsunobu reaction of allylic alcohols with o-nitrobenzenesulfonylhydrazine (NBSH) as nucleophile proceeds at −30 °C with invertive displacement; warming the resultant N-allylic sulfonylhydrazine derivative to 23 °C then leads to allylic diazene formation followed by sigmatropic elimination of dinitrogen. This one-step method for reductive 1,3-transposition is shown to be efficient and highly regio- and
-
Highly regioselective diels-alder reactions of 2-trimethylsilylmethyl- 1,3-butadiene catalyzed by a lewis acid and applications to syntheses of terpenes作者:Akira Hosomi、Hirokazu Iguchi、Jun-ichi Sasaki、Hideki SakuraiDOI:10.1016/s0040-4039(00)86886-0日期:1982.12-Trimethylsilylmethyl-1,3-butadiene undergoes highly regioselective Diels-Alder reactions with dienophiles such as acrolein and methyl vinyl ketone catalyzed by aluminum chloride in which the “para” isomers are obtained almost exclusively. The adducts are converted readily to a variety of naturally occurring mono and sesquiterpenes.
表征谱图
-
氢谱1HNMR
-
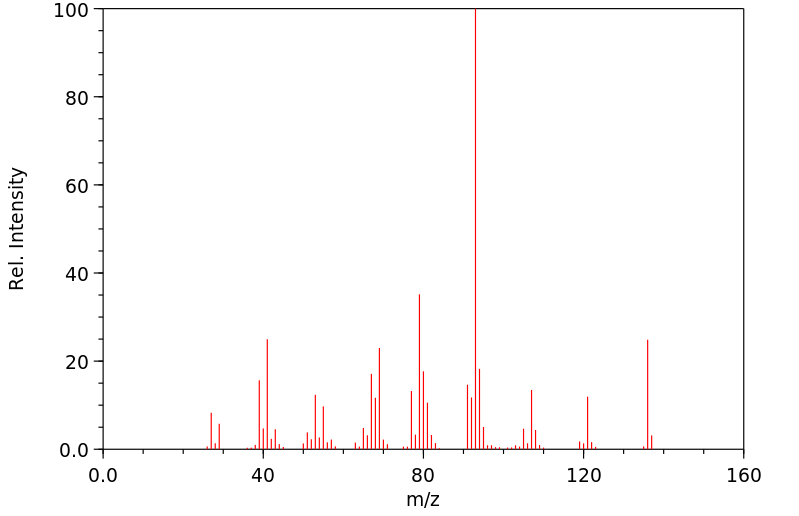
质谱MS
-
碳谱13CNMR
-
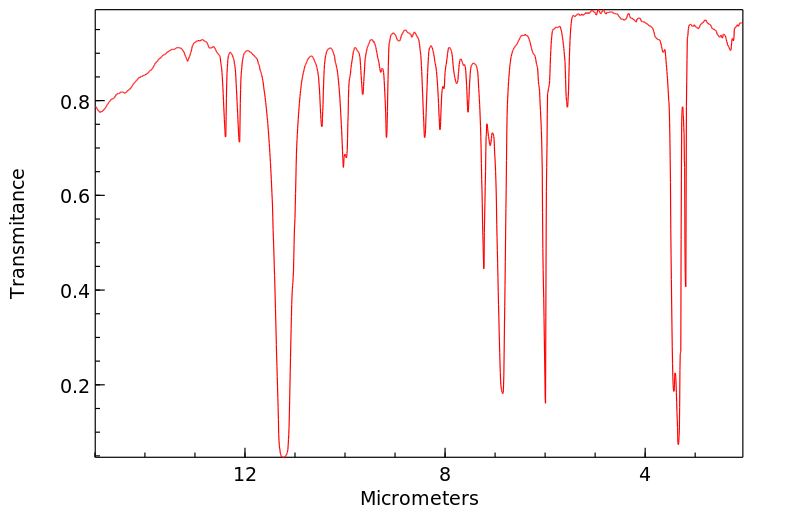
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸