甲基萘 | 90-12-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:34°C
-
沸点:229.78°C (estimate)
-
密度:1.0381 (estimate)
-
物理描述:1-methylnaphthalene is a colorless liquid. Freezing point -22°C (7.6°F). Boiling point 240-243°C (464-469°F). Flash point 82°C (180°F). Denser than water. Derived from coal tar and used in organic synthesis.
-
颜色/状态:Colorless liquid or oil
-
闪点:This chemical has a flash point of >93 °C (>200 °F). It is probably combustible.
-
溶解度:In water, 25.0 mg/L at 25 °C
-
蒸汽密度:4.91 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.067 mm Hg at 25 °C
-
亨利常数:5.14e-04 atm-m3/mole
-
大气OH速率常数:5.30e-11 cm3/molecule*sec
-
稳定性/保质期:
This chemical is stable under normal laboratory conditions. Solutions of this chemical in water, DMSO, 95% ethanol or acetone should be stable for 24 hours under normal lab conditions.
-
自燃温度:984 °F (529 °C)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes
-
汽化热:36.00 kJ/mol at 100.5 °C
-
气味阈值:THE ODOR THRESHOLD VALUE OF 1-METHYL NAPHTHALENE DETECTED @ ROOM TEMP & 60 DEG WAS 0.02 PPM.
-
折光率:Index of refraction: 1.6170 at 20 °C
-
碰撞截面:125.2 Ų [M+H]+ [CCS Type: DT, Method: stepped-field]
-
保留指数:1289.6;1289.6;1283;1293;1288.1;1286;1296;1285.68;1286.53;1287;1321;1272.66;1283.05;1289.57;1282;1330.12;1297.8;1318;1318;1318;1326;1300;1263;1268;1277;1278;1306;1326;1327;1268;1281;1303;1318;1282;1309;1295;1295;1298;1305;1302;1299;1289;1287.9;1328;1282;1298.4;1293;1290;1309;1321;1281;1301.7;1276;1273;1276;1277;1271;1282;1272;1278;1268;1288;1284;1289;1312;221;223.1
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
危险等级:9
-
危险品标志:Xn
-
安全说明:S61
-
危险类别码:R22,R51/53
-
WGK Germany:2
-
海关编码:29029080
-
危险品运输编号:UN 3082
-
包装等级:III
SDS
制备方法与用途
甲基萘是高温煤焦油加工产品之一,萘环上的一个氢原子被甲基取代,分为1-甲基萘(α-甲基萘)和2-甲基萘(β-甲基萘)两种异构体。主要从煤焦油及石油加工过程中的某些副产物中通过精馏分离得到。这两种化合物具有广泛的应用,如用作化工、医药和染料等工业的原料。
1-甲基萘对有机物有良好的溶解能力,可用作印染载体以及杀虫剂和抗鼠疫剂等溶剂;80%1-甲基萘与20%2-甲基萘的混合物为低共熔物,凝固点为-48℃,可用作热载体和溶剂。此外,1-甲基萘还可用于测定柴油十六烷值的标准燃料及精馏塔理论板数的试剂。
2-甲基萘可用于浸透剂、润滑油流动点降低剂以及溶剂等,并可生产治疗血管硬化药和饲料添加剂等。
理化性质 1-甲基萘无色油状液体,有近似萘的气味。能随蒸气一起挥发。相对密度1.0202,熔点-32℃,沸点244.6℃(在107.4℃时压力为1.333×10³Pa),折射率1.6170,闪点82℃。不溶于水,微溶于苯,易溶于乙醇和乙醚。
2-甲基萘从乙醇中析出者为无色单斜晶系结晶。相对密度1.0058,熔点34.58℃,沸点241℃(在104.7℃时压力为1.333×10³Pa),折射率1.6015(40℃)。闪点97℃。不溶于水,溶于苯,易溶于乙醇和乙醚。
有关甲基萘的概述、理化性质及应用是由Chemicalbook的丁红编辑整理。(2015-12-28)
制备方法 1-甲基萘的制备精馏洗油馏分从中切取馏程为240~245℃的甲基萘馏分,冷至-20℃后分离出粗2-甲基萘晶体。所得母液用于提取1-甲基萘。在母液中加入95~98%的浓硫酸磺化后,分离除去不磺化油质,再加水稀释,在10℃下析出1-甲基萘磺酸结晶。过滤后,在125~150℃下用过热蒸汽水解,除去水层即得纯度为85~90%的工业纯1-甲基萘。通过反复磺化、水解2~3次可获得纯度大于98%的1-甲基萘。
2-甲基萘的制备精馏洗油馏分从中切取馏程为240~245℃的甲基萘馏分,冷至-20℃后分离出粗2-甲基萘晶体。2-甲基萘粗品用乙醇或甲醇进行重结晶。所得晶体经离心分离和加热熔融过滤除去杂质,可得纯度在97%以上的2-甲基萘。
用途 1-甲基萘主要用作表面活性剂、减水剂、分散剂及药物等有机合成的原料;也可作为热载体和溶剂、硫磺提取剂以及生产增塑剂与纤维助染剂。此外,还可用于测定柴油十六烷值的标准燃料。
2-甲基萘主要用于生产维生素K3、聚酯纤维染色体载体、纤维助染剂、有机颜料、混凝土添加剂、洗涤剂、乳化剂、止血剂、润湿剂、植物生长调节剂(如1-萘乙酸)、饮料添加剂、饲料添加剂、口服避孕药和彩色胶卷染料等。
混合甲基萘上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,4-二甲基萘 1,4-dimethylnaphthalene 571-58-4 C12H12 156.227 1,5-二甲基萘 1,5-dimethylnaphthalene 571-61-9 C12H12 156.227 1,3-二甲基萘 1,3-dimethylnaphthalene 575-41-7 C12H12 156.227 1,8-二甲基萘 1,8-dimethylnaphthalene 569-41-5 C12H12 156.227 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 二甲基萘 dimethyl-1,2 naphtalene 573-98-8 C12H12 156.227 1-萘甲醇 1-naphthalene methanol 4780-79-4 C11H10O 158.2 1-萘甲醛 1-naphthaldehyde 66-77-3 C11H8O 156.184 1-(碘甲基)-萘 1-(iodomethyl)naphthalene 24471-54-3 C11H9I 268.097 1-乙烯萘 1-vinylnaphthalene 826-74-4 C12H10 154.211 (氯甲基)萘 1-Chloromethylnaphthalene 86-52-2 C11H9Cl 176.645 —— 1-mercaptomethylnaphthalene 5254-86-4 C11H10S 174.266 1-萘甲基胺 (naphth-1-yl)methylamine 118-31-0 C11H11N 157.215 1-溴甲基萘 2-(bromomethyl)naphthalene 3163-27-7 C11H9Br 221.096 氰基萘 1-Cyanonaphthalene 86-53-3 C11H7N 153.183 2,3-二甲基萘 2,3-dimethylnaphthalene 581-40-8 C12H12 156.227 —— <6-(3)H>-1-methylnaphthalene 22753-40-8 C11H10 144.192 (1-萘基甲基)甲基硫醚 (1-naphthylmethyl) methyl sulfide 5925-60-0 C12H12S 188.293 —— 1-naphthyldiazomethane 10378-55-9 C11H8N2 168.198 —— 1-naphtylaldehyde oxime —— C11H9NO 171.199 1-(二氟甲基)萘 1-(difluoromethyl)naphthalene 53731-26-3 C11H8F2 178.181 1-萘乙腈 1-Naphthylacetonitrile 132-75-2 C12H9N 167.21 1-烯丙基萘 1-allylnaphthalene 2489-86-3 C13H12 168.238 1-萘乙醇 2-naphthaleneethanol 773-99-9 C12H12O 172.227 惹烯 Retene 483-65-8 C18H18 234.341 二(5-甲氧基-2-{[(4-甲氧基-3,5-二甲基吡啶-2-基)甲基]亚硫酰基<亚磺酰>}苯并咪唑-1-化)二水合物镁 1,1'-dinaphthylmethane 28515-57-3 C21H16 268.358 1,2-二(1-萘基)乙烷 1,2-di(1-naphthyl)ethane 15374-45-5 C22H18 282.385 1-苯乙烯基萘 (E)-1-styrylnaphthalene 2840-87-1 C18H14 230.309 1-三氟甲基萘 1-trifluoromethylnaphthalene 26458-04-8 C11H7F3 196.172 —— 1-(2-methyl-1-propenyl)naphthalene 29926-68-9 C14H14 182.265 1-溴-4-甲基萘 1-bromo-4-methylnaphthalene 6627-78-7 C11H9Br 221.096 乙基 (1-萘基甲基)醚 (α-naphthylmethyl) ethyl ether 58530-15-7 C13H14O 186.254 2-溴-1-甲基萘 2-bromo-1-methylnaphthalene 20601-22-3 C11H9Br 221.096 (1-萘甲基)三氯硅烷 Trichlor-<(1-naphthyl)-methyl>lsilan 17998-59-3 C11H9Cl3Si 275.637 1-萘甲酸 1-naphthalenecarboxylic acid 86-55-5 C11H8O2 172.183 - 1
- 2
- 3
- 4
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-甲基苯并[A]蒽 5-methyltetraphene 2319-96-2 C19H14 242.32 —— 3,10-dimethyl-perylene 25889-65-0 C22H16 280.369 1,4-二甲基萘 1,4-dimethylnaphthalene 571-58-4 C12H12 156.227 2,9-/4,9-二甲基菲 2,9-dimethylphenanthrene 17980-09-5 C16H14 206.287 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 2,6-二甲基萘 2.6-dimethylnaphthalene 581-42-0 C12H12 156.227 二甲基萘 dimethyl-1,2 naphtalene 573-98-8 C12H12 156.227 2,7-二甲基萘 2,7-dimethylnaphthalene 582-16-1 C12H12 156.227 1-萘甲醇 1-naphthalene methanol 4780-79-4 C11H10O 158.2 1-萘甲醛 1-naphthaldehyde 66-77-3 C11H8O 156.184 1-(氟甲基)萘 1-(fluoromethyl)naphthalene 55831-10-2 C11H9F 160.191 1-(碘甲基)-萘 1-(iodomethyl)naphthalene 24471-54-3 C11H9I 268.097 三甲基萘 1,4,5-trimethylnaphthalene 2131-41-1 C13H14 170.254 1-乙基萘 1-ethylnapthelene 1127-76-0 C12H12 156.227 (氯甲基)萘 1-Chloromethylnaphthalene 86-52-2 C11H9Cl 176.645 1-萘甲基胺 (naphth-1-yl)methylamine 118-31-0 C11H11N 157.215 1-溴甲基萘 2-(bromomethyl)naphthalene 3163-27-7 C11H9Br 221.096 氰基萘 1-Cyanonaphthalene 86-53-3 C11H7N 153.183 抑芽醚 1-(methoxymethyl)naphthalene 5903-23-1 C12H12O 172.227 —— 1-Ethyl-4-methylnaphthalin 27424-87-9 C13H14 170.254 1-萘乙胺盐酸盐 2-naphthalen-1-yl-ethylamine 4735-50-6 C12H13N 171.242 (1-甲基萘-4-基)甲醇 4-methyl-1-naphthylmethanol 57322-44-8 C12H12O 172.227 —— 1-ethenyl-4-methylnaphthalene 85109-87-1 C13H12 168.238 —— 1-fluoromethyl-4-methylnaphthalene 79797-78-7 C12H11F 174.218 4-甲基-1-萘醛 4-methyl-1-naphthaldehyde 33738-48-6 C12H10O 170.211 1-氯甲基-4-甲基萘 1-(chloromethyl)-4-methylnaphthalene 5261-50-7 C12H11Cl 190.672 1-(二氯甲基)萘 1-(dichloromethyl)naphthalene 17180-26-6 C11H8Cl2 211.091 1-正丙基萘 1-n-propylnaphthalene 2765-18-6 C13H14 170.254 —— 1-naphtylaldehyde oxime —— C11H9NO 171.199 1-萘乙腈 1-Naphthylacetonitrile 132-75-2 C12H9N 167.21 (4-甲基-1-萘基)甲胺 (4-methylnaphthalen-1-yl)methanamine 771580-36-0 C12H13N 171.242 1-氰基-4-甲基萘 1-cyano-4-methylnaphthalene 36062-93-8 C12H9N 167.21 二溴甲基萘 1-Dibrommethyl-naphthalin 103502-51-8 C11H8Br2 299.993 1-烯丙基萘 1-allylnaphthalene 2489-86-3 C13H12 168.238 1-(溴甲基)-4-甲基萘 1-(bromomethyl)-4-methylnaphthalene 41791-10-0 C12H11Br 235.123 1,4-双(溴甲基)萘 1,4-bis(bromomethyl)naphthalene 58791-49-4 C12H10Br2 314.019 1-甲基萘-2-醇 1-methyl-2-naphthol 1076-26-2 C11H10O 158.2 1-甲基萘-2-甲醛 1-Methyl-2-naphthaldehyde 35699-45-7 C12H10O 170.211 1,2-二(1-萘基)乙烷 1,2-di(1-naphthyl)ethane 15374-45-5 C22H18 282.385 —— (E)-1,2-bis(1-naphthyl)ethene 1233-36-9 C22H16 280.369 1-苯乙烯基萘 (E)-1-styrylnaphthalene 2840-87-1 C18H14 230.309 4-氯-1-甲基萘 1-chloro-4-methylnaphthalene 17075-39-7 C11H9Cl 176.645 4-甲基-1-萘 4-methyl-1-naphthol 10240-08-1 C11H10O 158.2 —— 1-Methyl-2-fluoronaphthalene 703-47-9 C11H9F 160.191 1-溴-4-甲基萘 1-bromo-4-methylnaphthalene 6627-78-7 C11H9Br 221.096 5-甲基-1-萘酚 5-methylnaphthalen-1-ol 51149-87-2 C11H10O 158.2 2-溴-1-甲基萘 2-bromo-1-methylnaphthalene 20601-22-3 C11H9Br 221.096 1-甲基萘-2-甲腈 1-methyl-2-cyanonaphthalene 20176-06-1 C12H9N 167.21 —— 3-(naphthalen-1-yl)propan-1-ol 27653-22-1 C13H14O 186.254 —— 3-[1]naphthyl-acrylaldehyde 113388-92-4 C13H10O 182.222 1-(3-苯基丙基)萘 1-(3-phenylpropyl)naphthalene 29908-29-0 C19H18 246.352 1,1'-(1,3-丙烷二基)二萘 1,3-di-1-naphthylpropane 14564-86-4 C23H20 296.412 4-甲基萘-1-胺 1-amino-4-methylnaphthalene 4523-45-9 C11H11N 157.215 —— 3-(naphthalen-1-yl)propanal 53531-16-1 C13H12O 184.238 1-(3-溴丙基)萘 1-(3-bromopropyl)naphthalene 27650-86-8 C13H13Br 249.15 —— 4-Methyl-naphthalin-thiol-1 6383-53-5 C11H10S 174.266 1-碘-4-甲基萘 1-iodo-4-methylnaphthalene 70129-83-8 C11H9I 268.097 —— 1-Fluor-4-methyl-naphthalin 315-50-4 C11H9F 160.191 —— 1-(1-naphthyl)-2-(2-naphthyl)ethylene 2633-11-6 C22H16 280.369 —— 4-methoxymethyl-1-naphthylcarbinol 79996-87-5 C13H14O2 202.253 三甲基(萘-1-基甲基)硅烷 trimethyl(naphthalen-1-ylmethyl)silane 18410-58-7 C14H18Si 214.382 4-烯丙基-1-甲基萘 1-allyl-4-methylnaphthalene 23114-50-3 C14H14 182.265 —— 4-(naphthalen-1-yl)butan-1-ol 78396-13-1 C14H16O 200.28 1-(2,2,2-三氟乙基)萘 1-(2,2,2-trifluoroethyl)naphthalene 123228-02-4 C12H9F3 210.199 —— 1-benzyl-4-methylnaphthalene 51010-53-8 C18H16 232.325 8-甲基萘-1-胺 8-methylnaphthalen-1-amine 130523-30-7 C11H11N 157.215 —— 1-Methyl-6-acetylnaphthalin 24875-94-3 C13H12O 184.238 1-萘戊醛 5-(naphthalen-1-yl)pentanal 136415-75-3 C15H16O 212.291 5-(1-萘基)-戊-1-醇 5-(1-naphthyl)-pentan-1-ol 120756-49-2 C15H18O 214.307 1-萘甲酸 1-naphthalenecarboxylic acid 86-55-5 C11H8O2 172.183 - 1
- 2
- 3
- 4
- 5
- 6
- 7
反应信息
-
作为反应物:描述:甲基萘 在 palladium on activated charcoal 4-二甲氨基吡啶 、 氢氧化钾 、 N-溴代丁二酰亚胺(NBS) 、 lithium aluminium tetrahydride 、 过氧化氢苯甲酰 、 草酰氯 、 2-硝基丙烷 、 potassium tert-butylate 、 氢气 、 sodium methylate 、 二甲基亚砜 、 三乙胺 、 乙酰氯 、 lithium diisopropyl amide 作用下, 以 甲醇 、 乙醚 、 乙醇 、 二氯甲烷 、 苯 为溶剂, 反应 17.0h, 生成参考文献:名称:Synthesis and Biological Activity of New 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) Synthase Inhibitors: 2-Oxetanones with a Side Chain Mimicking the Folded Structure of 1233A.摘要:为模拟1233A(1)的折叠侧链构象,该化合物是一种3-羟基-3-甲基戊二酰辅酶A(HMG-CoA)合酶抑制剂,合成了侧链含有芳环的1233A类似物。其中2-恶坦酮部分保持不变。在1233A及其合成类似物中,反式-3-羟甲基-4-[2-(7-甲氧羰基-1-萘基)乙基]-2-恶坦酮(23)显示了最高的HMG-CoA合酶体外抑制活性。讨论了侧链上的构效关系。DOI:10.1248/cpb.42.512
-
作为产物:描述:magnesium,1-bromo-2-methanidylbenzene,bromide 在 bis-triphenylphosphine-palladium(II) chloride 、 potassium carbonate 作用下, 以 乙醚 、 乙醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 3.0h, 生成 甲基萘参考文献:名称:Ma, Shengming; Negishi, Ei-Ichi, Journal of the American Chemical Society, 1995, vol. 117, # 23, p. 6345 - 6354摘要:DOI:
-
作为试剂:描述:磷酸三丁酯 、 N,N,N,N-tetraethylammonium tetrafluoroborate 在 甲基萘 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 生成 phosphoric acid dibutyl ester ethyl ester 、 三乙胺参考文献:名称:Electrochemical reduction of triphenyl phosphate摘要:The electrolytic reduction of triphenyl phosphate proceeds with the participation of tetrabutylammonium cations with the formation of butyl diphenyl phosphate in DMF. It was concluded that the step involving electron transfer to the triphenyl phosphate molecule has retarded character.DOI:10.1007/bf00962373
文献信息
-
[EN] CYCLIC PEROXIDE OXIDATION OF AROMATIC COMPOUND PRODUCTION AND USE THEREOF<br/>[FR] OXYDATION DE PEROXYDE CYCLIQUE DE PRODUCTION DE COMPOSÉ AROMATIQUE ET SON UTILISATION申请人:UNIV TEXAS公开号:WO2014158209A1公开(公告)日:2014-10-02The present invention provides a method for converting an aromatic hydrocarbon to a phenol by providing an aromatic hydrocarbon comprising one or more aromatic C-H bonds and one or more activated C-H bonds in a solvent; adding a phthaloyl peroxide to the solvent; converting the phthaloyl peroxide to a di-radical; contacting the di-radical with the one or more aromatic C-H bonds; oxidizing selectively one of the one or more aromatic C-H bonds in preference to the one or more activated C-H bonds; adding a hydroxyl group to the one of the one or more aromatic C-H bonds to form one or more phenols; and purifying the one or more phenols.
-
Synthesis and spectroscopic properties of 1,4-diarylbutenynes作者:Alois H. A. Tinnemans、Wim H. LaarhovenDOI:10.1039/p29760001104日期:——Several new diarylbutenynes have been synthesized and analyses of the n.m.r. and u.v. spectra are described. For planar trans-isomers a conformational preference was found for 1-(α-naphthyl)- but not for 1-(β-naphthyl)-4-arylbutenynes. This difference is also found between compounds with C-1 attached to the α- or β-position of a larger aryl group.
-
Nondirected Copper-Catalyzed Sulfoxidations of Benzylic C–H Bonds作者:Hao Yu、Zhen Li、Carsten BolmDOI:10.1021/acs.orglett.8b00615日期:2018.4.6A copper-catalyzed sulfoxidation of benzylic C–H bonds by nondirected oxidative C(sp3)-H activation was developed. The process proceeds via sulfenate anions, which are generated by base-triggered elimination of β-sulfinyl esters and benzyl radicals. The functional group tolerance is high, and the product yields are good.
-
Copper-Catalyzed Late-Stage Benzylic C(sp3)–H Trifluoromethylation作者:Haiwen Xiao、Zhonglin Liu、Haigen Shen、Benxiang Zhang、Lin Zhu、Chaozhong LiDOI:10.1016/j.chempr.2019.02.006日期:2019.4Direct trifluoromethylation of C(sp3)–H bonds, especially in late stages, remains a formidable challenge. Herein, we describe the copper-catalyzed benzylic C(sp3)–H trifluoromethylation. With Cu(I) or Cu(II) as the catalyst, (bpy)Zn(CF3)2 (bpy = 2,2′-bipyridine) as the CF3 source, and NFSI (or Selectfluor) as the oxidant, site-selective benzylic C(sp3)–H trifluoromethylation is successfully implemented
-
[EN] GLYCOLATE OXIDASE INHIBITORS FOR THE TREATMENT OF DISEASE<br/>[FR] INHIBITEURS DE GLYCOLATE OXYDASE POUR LE TRAITEMENT D'UNE MALADIE申请人:BIOMARIN PHARM INC公开号:WO2020257487A1公开(公告)日:2020-12-24Described herein are compounds, methods of making such compounds, pharmaceutical compositions and medicaments containing such compounds, and methods of using such compounds to treat or prevent diseases or disorders associated with a defect in glyoxylate metabolism, for example a disease or disorder associated with the enzyme glycolate oxidase (GO) or alterations in oxalate metabolism. Such diseases or disorders include, for example, disorders of glyoxylate metabolism, including primary hyperoxaluria, that are associated with production of excessive amounts of oxalate.
表征谱图
-
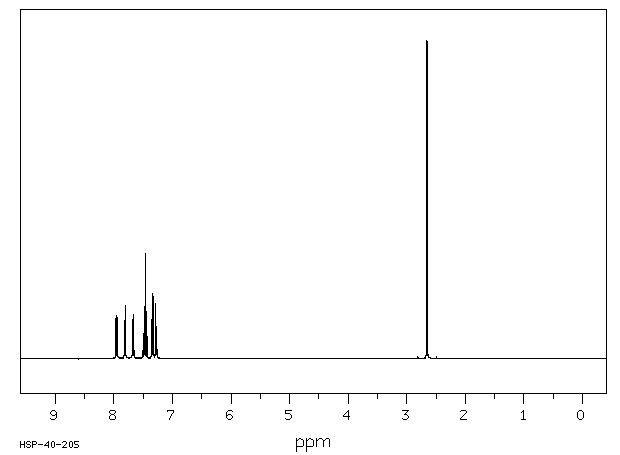
氢谱1HNMR
-
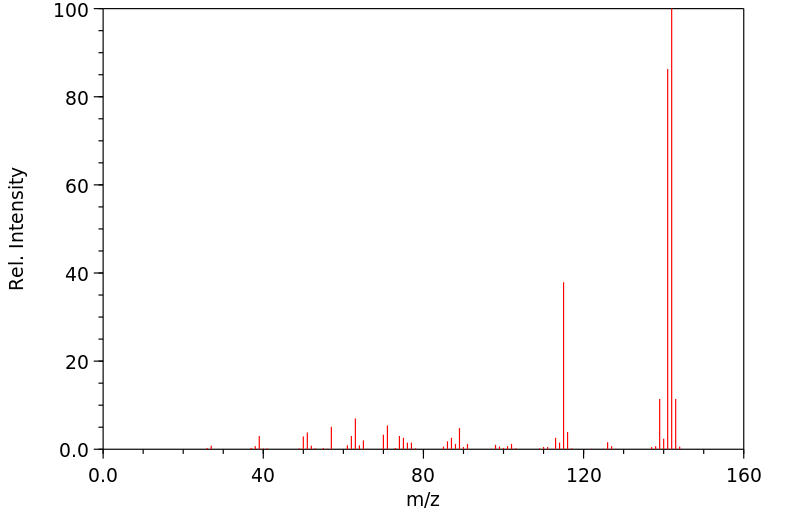
质谱MS
-
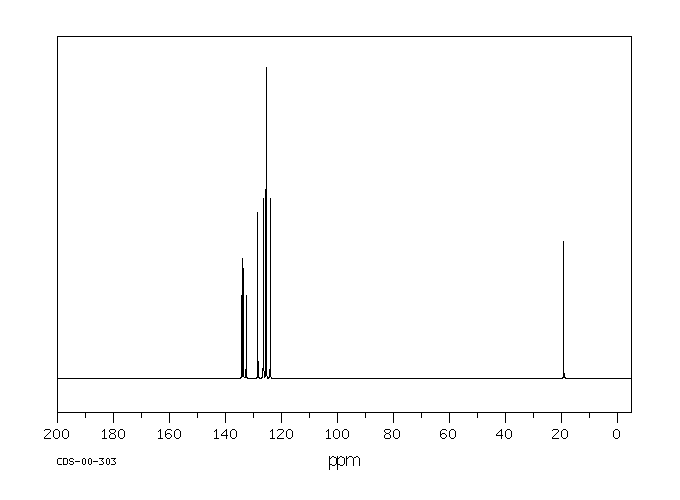
碳谱13CNMR
-
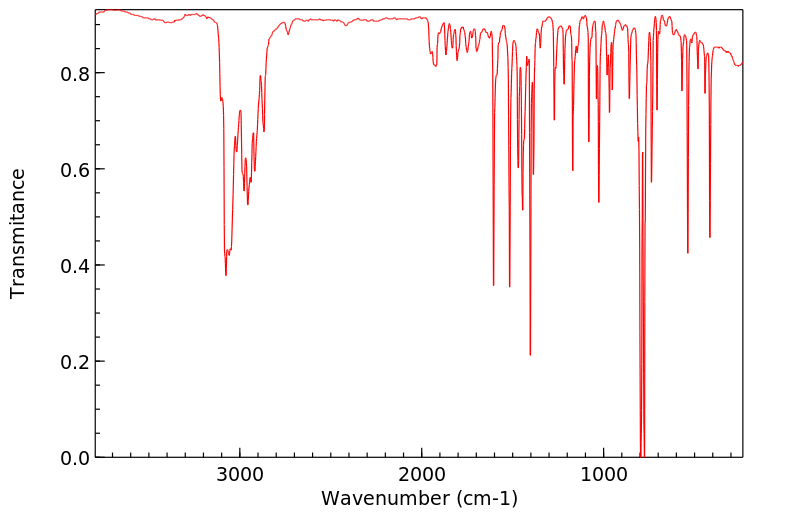
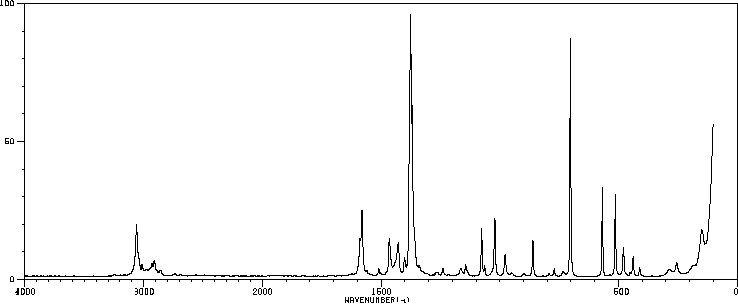
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息