2,6-二甲基萘 | 581-42-0
中文名称
2,6-二甲基萘
中文别名
——
英文名称
2.6-dimethylnaphthalene
英文别名
2,6-DMN;2,6-Dimethylnaphthalene
CAS
581-42-0
化学式
C12H12
mdl
MFCD00004120
分子量
156.227
InChiKey
YGYNBBAUIYTWBF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112 °C
-
沸点:262 °C
-
密度:1.000±0.06 g/cm3(Predicted)
-
溶解度:1.28e-05 M
-
蒸汽压力:0.00 mmHg
-
碰撞截面:126.3 Ų [M*]+; 128.6 Ų [M+H]+
-
保留指数:1381.69;1381.93;1382;1377;1400;1400;1401;1408;1402;1403;1387;1399;1377;1395.6;1408.8;1388;1403;1382;1367.7;1383.5;1384.5;1384;1388;1388;1389;1396;1388.3;1388;1400;237.6;239.7
-
稳定性/保质期:
存在于香料烟烟叶和烟气中。
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:N
-
安全说明:S22,S24/25
-
危险类别码:R50/53
-
WGK Germany:3
-
海关编码:2902909090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
2,6-二甲基萘 修改号码:5
模块 1. 化学品
产品名称: 2,6-Dimethylnaphthalene
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害
急性水生毒性 第2级
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述
对水生生物有毒性
防范说明
[预防] 避免释放到环境中。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,6-二甲基萘
百分比: >98.0%(GC)
CAS编码: 581-42-0
分子式: C12H12
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
2,6-二甲基萘 修改号码:5
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
111°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
2,6-二甲基萘 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2,6-二甲基萘 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2,6-Dimethylnaphthalene
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害
急性水生毒性 第2级
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述
对水生生物有毒性
防范说明
[预防] 避免释放到环境中。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,6-二甲基萘
百分比: >98.0%(GC)
CAS编码: 581-42-0
分子式: C12H12
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
2,6-二甲基萘 修改号码:5
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
111°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
2,6-二甲基萘 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2,6-二甲基萘 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
合成制备方法
- 烟草:OR,57/MS。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 2,7-二甲基萘 2,7-dimethylnaphthalene 582-16-1 C12H12 156.227 2,3-二甲基萘 2,3-dimethylnaphthalene 581-40-8 C12H12 156.227 1,6-二甲基萘 1,6-dimethylnaphthalene 575-43-9 C12H12 156.227 甲基萘 1-Methylnaphthalene 90-12-0 C11H10 142.2 2,6-双(羟基甲基)萘 naphthalene-2,6-dimethanol 5859-93-8 C12H12O2 188.226 2,6-双(溴甲基)萘 2,6-bis(bromomethyl)naphthalene 4542-77-2 C12H10Br2 314.019 二甲基萘 dimethyl-1,2 naphtalene 573-98-8 C12H12 156.227 1,5-二甲基萘 1,5-dimethylnaphthalene 571-61-9 C12H12 156.227 1,4-二甲基萘 1,4-dimethylnaphthalene 571-58-4 C12H12 156.227 6-甲基萘-2-醇 2-methyl-6-hydroxynaphthalene 17579-79-2 C11H10O 158.2 1,8-二甲基萘 1,8-dimethylnaphthalene 569-41-5 C12H12 156.227 2,6-萘二羧酸 2,6-Naphthalenedicarboxylic acid 1141-38-4 C12H8O4 216.193 6-甲酰基-2-萘甲酸 6-formyl-2-naphthoic acid 5084-45-7 C12H8O3 200.194 2-萘甲酸 2-Naphthoic acid 93-09-4 C11H8O2 172.183 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基萘 2-Methylnaphthalene 91-57-6 C11H10 142.2 2,7-二甲基萘 2,7-dimethylnaphthalene 582-16-1 C12H12 156.227 3,9-二甲基苯并[a]蒽 3,9-dimethylbenzanthracene 316-51-8 C20H16 256.347 3-甲基-苯并[a]蒽 3-methylbenz[a]anthracene 2498-75-1 C19H14 242.32 —— 3,10-dimethylbenzanthracene 63018-80-4 C20H16 256.347 1,6-二甲基萘 1,6-dimethylnaphthalene 575-43-9 C12H12 156.227 —— 6-methyl-2-naphthaldehyde 5084-46-8 C12H10O 170.211 (6-甲基萘-2-基)甲醇 (6-methylnaphthalene-2-yl)methanol 19182-14-0 C12H12O 172.227 —— 2-(bromomethyl)-6-methylnaphthalene 52988-15-5 C12H11Br 235.123 2,6-双(氯甲基)萘 2,6-bis(chloromethyl)naphthalene 93036-77-2 C12H10Cl2 225.117 2,6-双(溴甲基)萘 2,6-bis(bromomethyl)naphthalene 4542-77-2 C12H10Br2 314.019 —— 2-(chloromethyl)-(6-methyl)naphthalene 61187-17-5 C12H11Cl 190.672 1,7-二甲基萘 1,7-dimethylnaphthalene 575-37-1 C12H12 156.227 1,5-二甲基萘 1,5-dimethylnaphthalene 571-61-9 C12H12 156.227 6-甲基萘-2-醇 2-methyl-6-hydroxynaphthalene 17579-79-2 C11H10O 158.2 —— (6-methyl-[2]naphthyl)-acetonitrile 98245-84-2 C13H11N 181.237 1,8-二甲基萘 1,8-dimethylnaphthalene 569-41-5 C12H12 156.227 —— 2,6-bis-dibromomethyl-naphthalene 5859-91-6 C12H8Br4 471.812 —— 1,2-bis(6-methylnaphthalen-2-yl)ethane 43012-13-1 C24H22 310.439 五环[15.3.1.14,8.17,11.114,18]二十四-1(21),4(24),5,7,9,11(23),14(22),15,17,19-癸烯 <2.2> (2,6)naphthalenophane (achiral) 29041-32-5 C24H20 308.423 —— trans-2,6-distyrylnaphthalene 80236-22-2 C26H20 332.445 —— trans-2,6-Bis-<2-naphthyl-(2)-ethenyl>-naphthalin 80236-25-5 C34H24 432.565 —— trans 2-methyl-6-styrylnaphtalene 63216-65-9 C19H16 244.336 1,2,5,6-四甲基萘 1,2,5,6-tetramethylnaphthalene 2131-43-3 C14H16 184.281 —— 1,2,8-trimethylnaphthalene 3876-97-9 C13H14 170.254 2,6-萘二羧酸 2,6-Naphthalenedicarboxylic acid 1141-38-4 C12H8O4 216.193 6-甲酰基-2-萘甲酸 6-formyl-2-naphthoic acid 5084-45-7 C12H8O3 200.194 —— 6-methyl-2-naphthoic acid 5774-08-3 C12H10O2 186.21 2-甲氧基-6-甲基萘 2-methyl-6-methoxynaphthalene 26386-94-7 C12H12O 172.227 3,7-二甲基萘酚 3,7-dimethylnaphthol 69699-75-8 C12H12O 172.227 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:电有机反应。第56部分:2-甲基-和2-苄基萘的阳极氧化:影响竞争途径的因素摘要:系统地研究了在萘核的6位和苄基侧链的4-苯基位取代的2-甲基和2-苄基萘在亲核介质中的阳极氧化作用,以确定有利于环境的因素侧链取代。循环伏安法证实6-取代对萘核的氧化电位有深远的影响,13 C化学位移表明在苄基碳上有极性作用。然而,在任何尝试的条件下,几乎都没有观察到侧链阳极氧化。富电子底物的自由基阳离子优先二聚,并且在6位上具有强吸电子取代基(EtOSO 2)促进核替代。相反,在含水乙酸中用DDQ氧化可对富含电子的底物进行有效的侧链氧化,这与氢化物转移(可能在分子内通过电荷转移络合物)转移一致。DOI:10.1016/s0040-4020(02)00495-7
-
作为产物:描述:3-甲基-4-(对甲苯基)丁醛 在 iron(III) chloride 作用下, 以 1,1,2-三氯乙烷 为溶剂, 反应 2.0h, 以79%的产率得到2,6-二甲基萘参考文献:名称:WO2007/64105摘要:公开号:
-
作为试剂:描述:2,2,4,4-tetramethyl-3-thioxocyclobutanone S-oxide 、 二苯基甲烷硫酮 在 2,6-二甲基萘 作用下, 以 氘代氯仿 为溶剂, 反应 2.0h, 以100%的产率得到1,1,3,3-tetramethyl-7,7-diphenyl-6-oxa-5,8-dithiaspiro[3.4]octan-2-one参考文献:名称:1,3-偶极活性的脂肪族硫(,)(1)的环加成反应。摘要:2,2,4,4-四甲基-3-硫代氧杂环丁酮S-氧化物(4)在室温下与二芳基硫代酮结合,在平衡反应中提供螺1,1,2,4-恶二硫杂环戊烷6。化合物6a被氧化为顺式S,S-二氧化物9,其结构通过X射线分析确定。这些是具有烯丙基阴离子MO的硫酮S-氧化物(亚砜)的第一个明确的1,3-环加成;以前已经描述了将硫的C = S键的环加成为亲二亲性和亲二亲性。DOI:10.1021/jo9607424
文献信息
-
Decarbonylative Methylation of Aromatic Esters by a Nickel Catalyst作者:Toshimasa Okita、Kei Muto、Junichiro YamaguchiDOI:10.1021/acs.orglett.8b01233日期:2018.5.18A Ni-catalyzed decarbonylative methylation of aromatic esters was achieved using methylaluminums as methylating agents. Dimethylaluminum chlorides uniquely worked as the methyl source. Because of the Lewis acidity of aluminum reagents, less reactive alkyl esters could also undergo the present methylation. By controlling the Lewis acidity of aluminum reagents, a chemoselective decarbonylative cross-coupling
-
Tribromoisocyanuric Acid in Trifluoroacetic Acid: An Efficient System for Smooth Brominating of Moderately Deactivated Arenes作者:Marcio de Mattos、Pierre Esteves、Leonardo de AlmeidaDOI:10.1055/s-0032-1317795日期:——deactivated arenes are efficiently brominated by the reaction with tribromoisocyanuric acid (0.34 mol equiv) in trifluoroacetic acid at room temperature in 48–85% isolated yield. This medium avoids the polybromination of the substrate, observed in the same reaction performed in 98% H 2 SO 4 .
-
Cyclic Acetylenes. X. A Transannular Hypochromism Observed in a Cyclic Diacetylene Containing a Naphthalene Nucleus作者:Takashi Ando、Masazumi NakagawaDOI:10.1246/bcsj.40.363日期:1967.2A cyclic diacetylene (XIa) has been synthesized by the oxidative coupling of 1, 5-bis(propargyloxymethyl)naphthalene (X) with a cyclic dimer (XIb) and a cyclic trimer (XIc). The electronic spectra of XIb and XIc have been found to be almost identical with that of naphthalene with respect to the location of the absorption maxima and the intensities. On the other hand, a marked decrease in the absorption intensities has been observed in the spectrum of the cyclic monomer (XIa). An inspection of the Dreiding model of XIa indicates that the two conformations (A and B) can retain the maximum distance between the dyine unit and the naphthalene nucleus. On the basis of the molecular model, the distance between the two chromophores and the angle between the short axis of the nucleus and the bridging chain have been estimated to be 2.26 Å, 21° for A and 2.12 Åand 38° for B. Employing these data, the hypochromic effect exerted by the diyne chromophore on the absorption of the naphthalene nucleus has been calculated according to the theory of Tinoco and Rhodes, resulting in a fairly good agreement with the observed values. Inversely, the calculation of the distance and the angle between the two chromophoric groups using the observed hypochromic effect has also given reasonable values. Therefore, the hypochromism observed in the cyclic diacetylene (XIa) has been attributed to the operation of dispersion-force interaction between diyne unit and the naphthalene chromophore.通过1,5-双(丙炔氧甲基)萘(X)与环状二聚体(XIb)和环状三聚体(XIc)的氧化耦合反应,合成了一种环状二炔(XIa)。研究发现,XIb和XIc的电子光谱与萘的吸收最大位置和强度几乎相同。另一方面,在环状单体(XIa)的光谱中观察到了明显的吸收强度下降。对XIa的Dreiding模型的检查表明,两种构象(A和B)能够保持二炔单元和萘核之间的最大距离。基于分子模型,估计了两个发色团之间的距离和核短轴与桥链之间的角度,分别为A的2.26 Å和21°,B的2.12 Å和38°。利用这些数据,根据Tinoco和Rhodes的理论计算了二炔发色团对萘核吸收的减色效应,结果与观察值相当一致。相反,使用观察到的减色效应计算两个发色团之间的距离和角度也给出了合理值。因此,观察到的环状二炔(XIa)的减色效应归因于二炔单元和萘发色团之间的色散力相互作用。
-
Activation of Bismuth(III) Derivatives in Ionic Liquids: Novel and Recyclable Catalytic Systems for Friedel−Crafts Acylation of Aromatic Compounds作者:Said Gmouh、Hongli Yang、Michel VaultierDOI:10.1021/ol034529n日期:2003.6.1The activity of four bismuth(III) derivatives when employed as Friedel-Crafts catalysts for the acylation of aromatics was found to increase dramatically when dissolved in ionic liquids. Solutions of bismuth oxide or triflate in [emim][NTf(2)] and [bmim][NTf(2)] are the most efficient catalytic systems, with catalyst loading as low as 1% leading to clean, high-yielding acylation of a variety of benzene
-
Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes Catalyzed by a Brønsted Acid in Hexafluoroisopropanol作者:Edward Richmond、Vuk D. Vuković、Joseph MoranDOI:10.1021/acs.orglett.7b03688日期:2018.2.2A general, Brønsted acid catalyzed method for the room temperature, nucleophilic ring opening of donor–acceptor cyclopropanes in fluorinated alcohol solvent, HFIP, is described. Salient features of this method include an expanded cyclopropane scope, including those bearing single keto-acceptor groups and those bearing electron-deficient aryl groups. Notably, the catalytic system proved amenable to
表征谱图
-
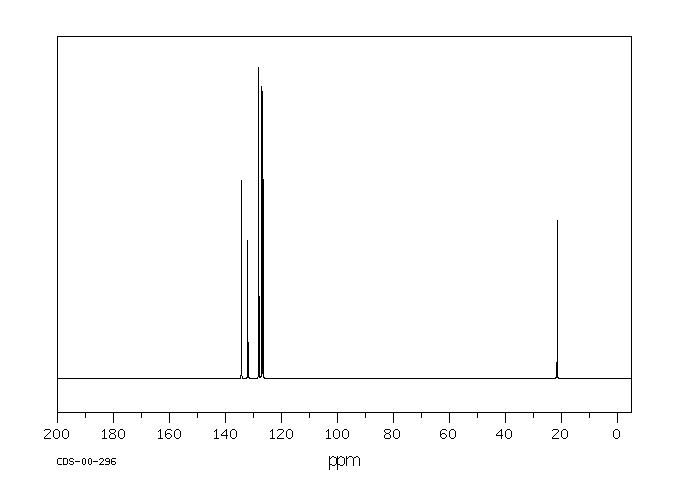
氢谱1HNMR
-
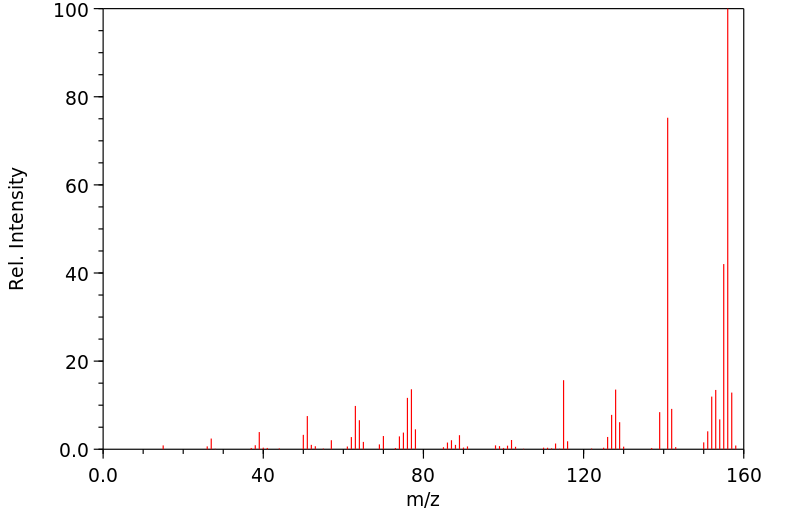
质谱MS
-
碳谱13CNMR
-
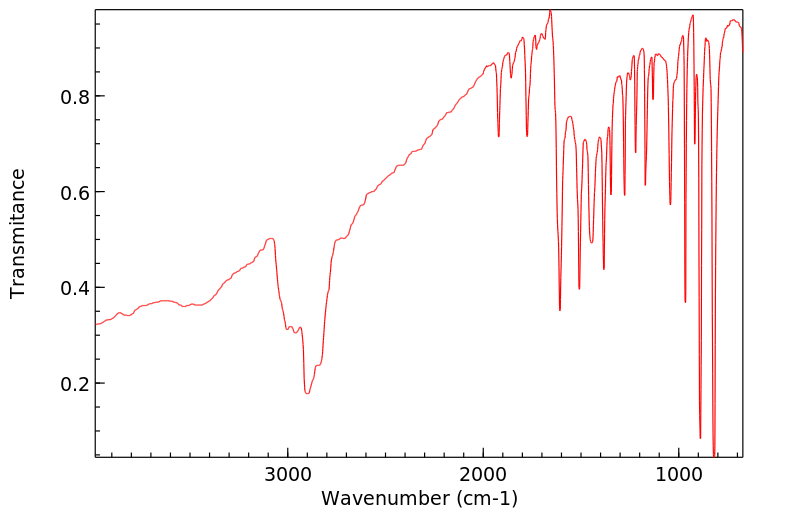
红外IR
-
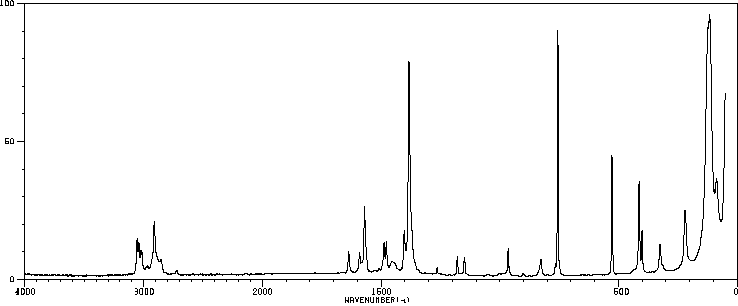
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮