1,4-双(溴甲基)萘 | 58791-49-4
中文名称
1,4-双(溴甲基)萘
中文别名
1,4-双(溴甲基)奈;1,4-双(溴甲基)萘(含约23%的异构体)
英文名称
1,4-bis(bromomethyl)naphthalene
英文别名
1,4-di(bromomethyl)naphthalene;1,4-dimethylnaphtalene-α,α'-dibromide
CAS
58791-49-4
化学式
C12H10Br2
mdl
——
分子量
314.019
InChiKey
UZHZQZOMHXNQBJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:169.0 to 173.0 °C
-
沸点:180 °C(Press: 1 Torr)
-
密度:1.720±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与水分接触。
计算性质
-
辛醇/水分配系数(LogP):4.8
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:8
-
海关编码:2903999090
-
危险品运输编号:UN 1759
-
储存条件:在密封的贮藏器中,并将其存放在阴凉、干燥的地方。
SDS
1,4-Bis(bromomethyl)naphthalene (contains ca. 23%
isomer)
SAFETY DATA SHEET
Section 1. IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIER
Product name: 1,4-Bis(bromomethyl)naphthalene (contains ca. 23% isomer)
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS
Corrosive to metals Category 1
HEALTH HAZARDS
Skin corrosion/irritation Category 1C
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Danger
Signal word
Hazard statement May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements
Keep only in original container.
[Prevention]
Do not breathe.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
23% isomer)
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 1,4-Bis(bromomethyl)naphthalene (contains ca. 23% isomer)
Percent: >98.0%(T)
CAS Number: 58791-49-4
Chemical Formula: C12H10Br2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Use corrosive resistant equipment.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed. Keep only in original container.
23% isomer)
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
White - Very pale yellow
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:178 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
23% isomer)
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
3261
UN-No:
Proper shipping name: Corrosive solid, acidic, organic, n.o.s.
III
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
isomer)
SAFETY DATA SHEET
Section 1. IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIER
Product name: 1,4-Bis(bromomethyl)naphthalene (contains ca. 23% isomer)
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS
Corrosive to metals Category 1
HEALTH HAZARDS
Skin corrosion/irritation Category 1C
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Danger
Signal word
Hazard statement May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements
Keep only in original container.
[Prevention]
Do not breathe.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
23% isomer)
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 1,4-Bis(bromomethyl)naphthalene (contains ca. 23% isomer)
Percent: >98.0%(T)
CAS Number: 58791-49-4
Chemical Formula: C12H10Br2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Use corrosive resistant equipment.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed. Keep only in original container.
23% isomer)
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
White - Very pale yellow
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:178 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
23% isomer)
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
3261
UN-No:
Proper shipping name: Corrosive solid, acidic, organic, n.o.s.
III
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-(溴甲基)-4-甲基萘 1-(bromomethyl)-4-methylnaphthalene 41791-10-0 C12H11Br 235.123 1,4-二甲基萘 1,4-dimethylnaphthalene 571-58-4 C12H12 156.227 甲基萘 1-Methylnaphthalene 90-12-0 C11H10 142.2 4-甲氧基甲基-1-萘基甲醇 1-acetyl-4-bromomethylnaphthalene 79996-80-8 C13H11BrO 263.134 1,4-萘二甲醇 (naphthalene-1,4-diyl)dimethanol 57322-45-9 C12H12O2 188.226 1-氯甲基-4-甲基萘 1-(chloromethyl)-4-methylnaphthalene 5261-50-7 C12H11Cl 190.672 1,4-双氯甲基萘 1,4-bis(chloromethyl)naphthalene 6586-89-6 C12H10Cl2 225.117 —— 4-methoxymethyl-1-naphthylcarbinol 79996-87-5 C13H14O2 202.253 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,4-bis-dibromomethyl-naphthalene 104036-75-1 C12H8Br4 471.812 —— 1,4-Naphthalindimethanthiol 59045-58-8 C12H12S2 220.359 —— 1,4-bis(aminomethyl)naphthalene 108629-58-9 C12H14N2 186.257
反应信息
-
作为反应物:描述:1,4-双(溴甲基)萘 在 sodium azide 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 以100%的产率得到1,4-bis(azidomethyl)naphthalene参考文献:名称:Generation and in Situ Evaluation of Libraries of Poly(acrylic acid) Presenting Sialosides as Side Chains as Polyvalent Inhibitors of Influenza-Mediated Hemagglutination摘要:This paper describes a simple, microscale method for generating and evaluating libraries of derivatives of poly(acrylic acid) (pAA) that present mixtures of side chains that influence their biological activity. The method is based on the one-step conversion of poly(acrylic anhydride) (pAAn) to linear polymers presenting multiple units of R on side chains, pAA(R): the polymers are obtained by ultrasonication of a suspension of pAAn and aqueous RNH2 contained in a 250-mu L well of a microtiter plate. Using this method, derivatives of pAA having N-acetylneuraminic acid (NeuAc-L-NH2) as a side chain, pAA(NeuAc-L), were generated and assayed for ability to inhibit hemagglutination (HAI) of chicken erythrocytes by influenza virus A (X-31); the constant (KHAT) describing this inhibition is calculated on the basis of the concentration of NeuAc groups in solution, rather than the concentration of polymer molecules. Go-polymeric pAA(NeuAc-L-n; L-n=different linking groups) with a range of mole fractions of NeuAc-L-NH2 (chi(NeuAc-L)=0.02-0.11) exhibited HAI activities with K-i(HAI) values between 27 and 0.30 mu M. Using combinations of NeuAc-L-NH2 and one of 26 different primary amines RNH2, a variety of ter-polymeric pAA(NeuAc-L; R) (chi(Neu-Ac-L)similar to 0.05; chi(R) similar to 0.06) were also generated and assayed. Certain ter-polymers yielded values of K-i(HAI) that were lower by a factor of similar to 10(4) than that of the parent co-polymeric pAA(NeuAc-L): the most active inhibitor was pAA(NeuAc-L; L-3-(2'-naphthyl)alanine)) (K(i)(HAI)approximate to 0.05 mM). Typically, the incorporation of hydrophobic-especially aromatic-side chains enhanced activities. These polymers (pAA(NeuAc-L; R)) belong to a new class of polymeric, polyvalent sialosides that are potent inhibitors of the adsorption of influenza virus to erythrocytes. They were active with only low to moderate levels of incorporation of functional groups into the side chains: chi(NeuAc-L)similar to 0.05; chi(R) similar to 0.06.DOI:10.1021/ja963519x
-
作为产物:参考文献:名称:Saint-Juan,R.; Canonne,P., Bulletin de la Societe Chimique de France, 1971, p. 3330 - 3335摘要:DOI:
文献信息
-
New Synthetic Method of [2.2]Cyclophanes via Diselena[3.3]cyclophanes作者:Hiroyuki Higuchi、Keita Tani、Tetsuo Otsubo、Yoshiteru Sakata、Soichi MisumiDOI:10.1246/bcsj.60.4027日期:1987.11Syntheses of cyclic diselenides through diselenolates and the following deselenation reactions by means of three ways were studied. The preparation of the cyclic diselenides in the presence of excess sodium borohydride gave thirty-eight diselenides containing diselena[3.3]cyclophanes and alicyclic diseleno compounds in high yields in addition to two triple-bridged selenacyclophanes. Photodeselenation
-
New carbamoylpiperidines as human platelet aggregation inhibitors作者:Zhengming Guo、Xiaozhang Zheng、Walter Thompson、Marion Dugdale、Ram GollamudiDOI:10.1016/s0968-0896(00)00033-x日期:2000.5series of 3-carbamoylpiperidines (nipecotamides) are designed, synthesized and tested for their inhibitory action against adenosine diphosphate (ADP)-induced aggregation of human platelets. A structure-activity analysis of the bis(nipecotamido)aralkane type showed that a substituent on the piperidine ring should preferably be an amide and that the electronegativity of the carbonyl oxygen and the orientation设计,合成和测试了一系列3-氨基甲酰基哌啶(邻甲酰胺)对二磷酸腺苷(ADP)诱导的人血小板聚集的抑制作用。对双(邻氨基苯甲酰氨基)芳烷类型的结构活性分析表明,哌啶环上的取代基应优选为酰胺,并且羰基氧的电负性和酰胺基的取向影响活性。基于影响血小板活化和聚集的因素的知识,可在原位释放一氧化氮(NO)的硝酸酯部分被掺入到烟酰胺结构中。与没有-ONO2功能的那些化合物相比,这些化合物显示出增强的活性。它们还表现出立体选择性,内消旋异构体的效力约为合成非对映异构体混合物的两倍。用羟基,酯或烷基取代-ONO2的功能大大降低了抑制聚集的能力。此处显示的烟酰胺可抑制基础和胶原蛋白诱导的血小板三磷酸肌醇(IP3)水平以及磷酸肌醇周转率的升高。考虑到早期的结果,提出了一种综合的行动机制。
-
Linked cyclic polyamines with activity against hiv.申请人:AnorMED Inc.公开号:EP1223166A1公开(公告)日:2002-07-17There is disclosed a pharmaceutical composition comprising as active ingredient a linked cyclic compound of general formula I, Z - R - A - R' - Y (I) in which Z and Y are independently cyclic polyamine moieties having from 9 to 32 ring members and from 3 to 8 amine nitrogens in the ring spaced by 2 or more carbon atoms from each other, A is an aromatic or heteroaromatic moiety, R and R' are each a substituted or unsubstituted alkylene chain or heteroatom containing chain. or an acid addition salt or metal complex thereof, in admixture or association with a pharmaceutically acceptable diluent or carrier.
-
Polymeric chiral phase-transfer catalysts derived from cinchona alkaloids for enantioselective synthesis of α-amino acids作者:Jeong-Hee Lee、Mi-Sook Yoo、Ji-Hee Jung、Sang-sup Jew、Hyeung-geun Park、Byeong-Seon JeongDOI:10.1016/j.tet.2007.05.076日期:2007.8practical chiral phase-transfer catalysts (PTCs). Presented are the details on the development of the dimeric PTCs for the synthesis of optically active α-amino acid derivatives and the optimization of the reaction variables suitable for the dimeric PTCs. The 1,3-phenyl- and the 2,7-naphthyl-linked dimeric PTCs showed excellent catalytic capability on the reactivity and enantioselectivity in the catalytic
-
Self-Assembly of Small Peptidomimetic Cyclophanes作者:Jorge Becerril、M. Isabel Burguete、Beatriu Escuder、Francisco Galindo、Raquel Gavara、Juan F. Miravet、Santiago V. Luis、Gabriel PerisDOI:10.1002/chem.200400031日期:2004.8.20The self-assembly of a series of small peptidomimetic cyclophanes in organic solvents was studied. X-ray diffraction, NMR spectroscopy, and molecular modelling were used to understand the structural features of these self-assembling compounds both at the molecular and supramolecular level. The factors that could influence the formation of gels rather than crystals were studied and a model for the arrangement研究了一系列小拟肽环烷在有机溶剂中的自组装。使用X射线衍射,NMR光谱和分子模型来了解这些自组装化合物在分子和超分子水平上的结构特征。研究了可能影响凝胶而不是晶体形成的因素,并提出了分子在凝胶中排列的模型。此外,扫描电子显微镜显示,在某些情况下,这些化合物在从有机胶凝剂转变为螺旋状凝胶纤维时会经历手性的转录。
表征谱图
-
氢谱1HNMR
-
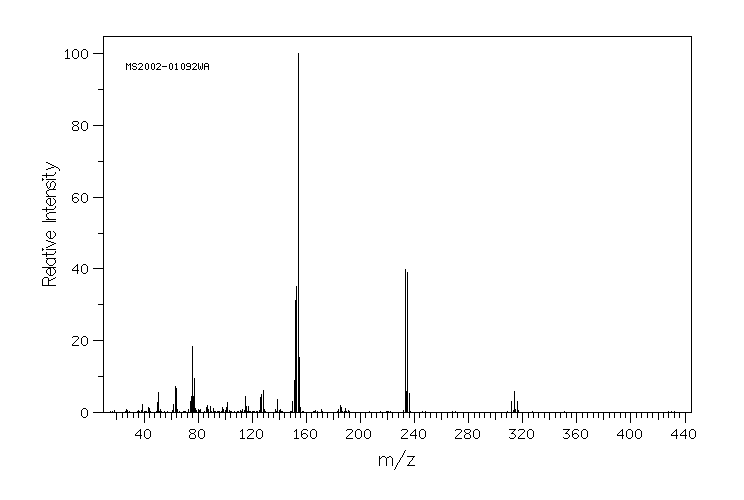
质谱MS
-
碳谱13CNMR
-
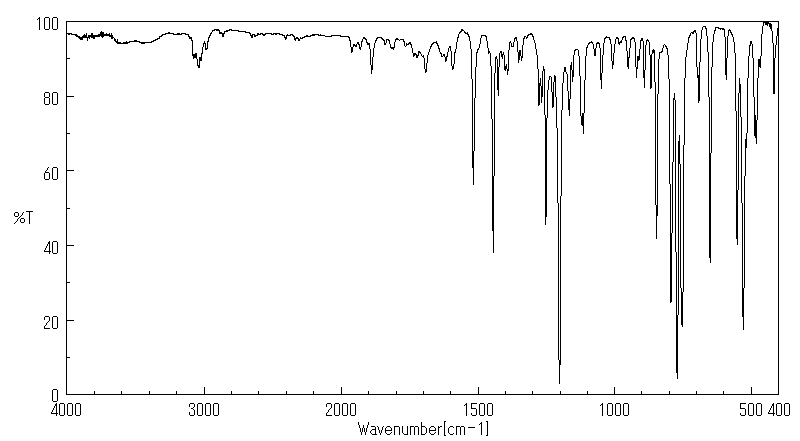
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮