4-硝基甲苯 | 99-99-0
中文名称
4-硝基甲苯
中文别名
对硝基甲苯;1-甲基-4-硝基苯
英文名称
1-methyl-4-nitrobenzene
英文别名
4-Nitrotoluene;p-nitrotoluene;4-methylnitrobenzene;para-nitrotoluene;4-NT;p-methylnitrobenzene;PNT
CAS
99-99-0
化学式
C7H7NO2
mdl
MFCD00007366
分子量
137.138
InChiKey
ZPTVNYMJQHSSEA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:52-54 °C (lit.)
-
沸点:238 °C (lit.)
-
密度:1.392 g/mL at 25 °C (lit.)
-
蒸气密度:4.7 (vs air)
-
闪点:223 °F
-
溶解度:水中的溶解度0.26克/升
-
介电常数:22.2(58.0℃)
-
暴露限值:NIOSH REL: TWA 2 ppm (11 mg/m3), IDLH 200 ppm; OSHA PEL: TWA 5 ppm (30 mg/m3); ACGIH TLV: TWA 2 ppm (adopted).
-
LogP:2.37 at 25℃
-
物理描述:P-nitrotoluene appears as a yellow liquid with a weak aromatic odor. Toxic. Insoluble in water. Combustible but may take some effort to ignite. Produces toxic oxides of nitrogen when burned. In a spill, immediate steps should be taken to limit its spread to the environment. Can easily penetrate the soil and contaminate groundwater or nearby streams. Used to make other chemicals.
-
颜色/状态:Yellowish crystals
-
气味:Bitter almond
-
蒸汽密度:4.72 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.0157 mm Hg at 25 °C /extrapolated/
-
亨利常数:5.63e-06 atm-m3/mole
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、强还原剂、强碱。
-
避免接触的条件:受热。
-
聚合危害:不聚合。
-
分解产物:氮氧化物。
-
-
自燃温度:Auto-ignition temperature: 450 °C
-
分解:Decomposes violently at 279 °C and will burn even in absence of air.
-
粘度:1.2 mPa (= cP) at 60 °C
-
燃烧热:897.0 kg.cal at 20 °C (liquid); 888.6 kg.cal at 20 °C (solid)
-
汽化热:11,915.0 gram cal/gram mole
-
表面张力:42.3 mN/m (= dyn/cm) at 60 °C
-
电离电位:9.50 eV
-
折光率:Index of refraction: 1.5382 at 15 °C
-
保留指数:1167;1170;1195;1212;1199.3
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
代谢
Yields p-nitrobenzyl alcohol in rabbits. ...Yields p-nitrobenzyl alcohol in rats and mice ... . /from table/
来源:Hazardous Substances Data Bank (HSDB)
代谢
鳗鱼肝脏匀浆对p-硝基甲苯甲基团的代谢速度比大鼠肝脏匀浆慢。
Eel liver homogenates metabolize methyl group of p-nitrotoluene, model compound of toluene, more slowly than rat liver homogenates.
来源:Hazardous Substances Data Bank (HSDB)
代谢
脊椎动物肝脏酶和完整的草蛴螬以不同的速率将p-硝基甲苯的甲基团氧化成羧基团。
Vertebrate liver enzymes and intact grass grubs oxidized methyl groups of p-nitrotoluene into carboxy groups at different rates.
来源:Hazardous Substances Data Bank (HSDB)
代谢
2-、3-和4-硝基甲苯(分别简称2NT、3NT和4NT)在雄性Fischer 344大鼠体内的代谢和排泄进行了研究。... 在给予4NT后72小时内,尿液中排出的主要代谢物是4-硝基苯甲酸(占剂量的28%)、4-乙酰胺基苯甲酸(占剂量的27%)和4-硝基马尿酸(占剂量的13%)。
The metabolism and excretion of 2-, 3-, and 4-nitrotoluene (2NT, 3NT, and 4NT, respectively) were studied in male Fischer 344 rats. ... The major metabolites excreted in urine in 72 hr after administration of 4NT were 4-nitrobenzoic acid (28% of the dose), 4-acetamidobenzoic acid (27% of the dose), and 4-nitrohippuric acid (13% of the dose).
来源:Hazardous Substances Data Bank (HSDB)
代谢
在给予4-硝基甲苯后,尿液中发现八种代谢物。对于三只大鼠处理72小时后,每种代谢物的平均给药剂量百分比加上或减去标准误的平均值分别为:4-硝基苯甲酸(28.0 +/- 2.6);4-乙酰胺基苯甲酸(27.1 +/- 3.0);4-硝基马尿酸(13.0 +/- 0.7);S-(4-硝基苄基)-N-乙酰半胱氨酸(3.7 +/- 0.1);4-硝基苄基葡萄糖醛酸苷(1.4 +/- 0.1);4-氨基苯甲酸(0.8 +/- 0.1);S-甲基-2-硝基苯基葡萄糖醛酸苷(0.3 +/- 0.0);以及5-甲基-2-硝基苯基硫酸盐(0.2 +/- 0.0)。
Eight metabolites were found in urine following administration of 4-nitrotoluene. ... For three rats 72 hr after treatment, the mean percentage of the dose administered plus or minus the standard error the mean for each metabolite was: 4-nitrobenzoic acid (28.0 +/- 2.6); 4-acetamidobenzoic acid (27.1 +/- 3.0); 4-nitrohippuric acid (13.0 +/- 0.7); S-(4-nitrobenzyl)-N-acetylcysteine (3.7 +/- 0.1); 4-nitrobenzyl glucuronide (1.4 +/- 0.1); 4-aminobenzoic acid (0.8 +/- 0.1); S-methyl-2- nitrophenyl glucuronide (0.3 +/- 0.0); and 5-methyl-2-nitrophenyl sulfate (0.2 +/- 0.0)
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Evaluation: There is inadequate evidence in humans for the carcinogenicity of nitrotoluenes. There is inadequate evidence in experimental animals for the carcinogenicity of ... 4-nitrotoluene. ... Overall evaluation: Nitrotoluenes are not classifiable as to their carcinogenicity to humans (Group 3).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:硝基甲苯
IARC Carcinogenic Agent:Nitrotoluenes
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:无法归类其对人类致癌性
IARC Carcinogenic Classes:Group 3: Not classifiable as to its carcinogenicity to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
IARC Monographs:Volume 65: (1996) Printing Processes and Printing Inks, Carbon Black and Some Nitro Compounds
来源:International Agency for Research on Cancer (IARC)
毒理性
该物质可以通过吸入其气溶胶、通过皮肤接触以及摄入进入人体。
The substance can be absorbed into the body by inhalation of its aerosol, through the skin and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
2-、3-和4-硝基甲苯(分别简称2NT、3NT和4NT)在Fischer 344雄性大鼠体内的代谢和排泄被研究。在通过口服剂量(每公斤200毫克)给予放射性标记的2NT、3NT或4NT之后,收集了72小时的排泄物。每个硝基甲苯异构体的放射性标记都被迅速排泄(2NT、3NT和4NT在24小时内分别排泄了剂量的86、73和74%)。尿液是主要的排泄途径,72小时内通过该途径排泄了70-85%的剂量。在72小时内,5至13%和0.0至0.1%的剂量分别通过粪便和呼出气体排泄...
The metabolism and excretion of 2-, 3-, and 4-nitrotoluene (2NT, 3NT, and 4NT, respectively) were studied in male Fischer 344 rats. Excreta were collected for 72 hr after an oral dose (200 mg/kg) of radiolabeled 2NT, 3NT, or 4NT. Radiolabel from each NT isomer was rapidly excreted (86, 73 and 74% of the dose for 2NT, 3NT, and 4NT, respectively in 24 hr). The urine was the major route of excretion with 70-85% of the dose being excreted by that route in 72 hr. Five to 13% and 0.0 to 0.1% of the dose was excreted in the feces and expired air, respectively, in 72 hr. ...
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
该物质可以通过吸入其气溶胶、通过皮肤接触以及摄入进入人体。
The substance can be absorbed into the body by inhalation of its aerosol, through the skin and by ingestion
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在大鼠单次给药14C标记的硝基甲苯后,约77%在72小时内通过尿液排出...
After a single dose of (14)C-labeled 4-nitrotoluene to male rats, about 77% was excreted in the urine within 72 hr... .
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
In bile duct-cannulated male Fischer 344 rats administered 200 mg/kg bw nitro (14)C toluene isomers ... 9.8% of the 4-nitrotoluene dose was excreted in bile, compared to 1.3% in females.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 2 ppm (11 mg/m3) [skin]
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T,N
-
安全说明:S16,S27,S28,S28A,S37,S45,S61
-
危险类别码:R23/24/25,R51/53,R33
-
WGK Germany:2
-
海关编码:29042000
-
危险品运输编号:UN 3446 6.1/PG 2
-
危险类别:6.1
-
RTECS号:XT3325000
-
包装等级:II
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,包装密封。 - 应与氧化剂、还原剂、碱类及食用化学品分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储区应备有合适的材料收容泄漏物。
制备方法与用途
对硝基甲苯 (PNT)
对硝基甲苯(PNT),又称为p-硝基甲苯或4-硝基甲苯,是一种浅黄色晶体,具有特有的苦杏仁气味,几乎不溶于水。它主要用于生产DSD酸、增白剂,并用作染料、农药、医药和合成纤维的中间体,也能作为爆炸品检测的示踪剂。
制备对硝基甲苯常以浓硝酸为硝化剂、浓硫酸为催化剂,通过甲苯的硝化反应制备。一种新的实验方法是使用发烟硝酸为硝化剂和固体NaHSO₄作为可循环使用的催化剂,在CCl₄溶液中加入乙酸酐(有脱水作用),在45℃下反应1小时。反应结束后,过滤滤液,并分别用5% NaHCO₃溶液和水洗涤至中性,再经分离提纯得到对硝基甲苯。
化学性质对硝基甲苯为黄色斜方六面晶体,不溶于水,易溶于乙醇、乙醚和苯。
用途对硝基甲苯广泛用于制造对甲苯胺、甲苯二异氰酸酯等,也是农药、染料、医药、塑料和合成纤维助剂的中间体。它还是除草剂绿麦隆等多种化学品的中间体,并可以制造多种衍生物如对硝基苯甲酸、对硝基甲苯-2-磺酸、2-硝基-4-甲基苯胺等,同时也用于医药和染料生产。
生产方法甲苯用混酸进行硝化反应后分离得到对硝基甲苯。制备方法是将甲苯加入硝化反应釜中,在25℃以下冷却,并加入配好的混酸(硝酸25%~30%,硫酸55%~58%,水20%~21%),继续搅拌并控制温度不超过50℃,约1~2小时后结束反应。然后静置6小时,分离生成的硝基苯,用清水和碱液洗涤,粗制的对硝基甲苯中含有邻硝基甲苯、对硝基甲苯和间硝基甲苯。经过真空蒸馏分离大部分邻硝基甲苯,残余含有较多对硝基甲苯的馏分再经减压蒸馏分出,并通过冷却结晶分离最终得到纯品。
类别- 类别:有毒物品
- 毒性分级:中毒
- 急性毒性:大鼠口服LD₅₀: 1960 毫克/公斤;小鼠口服LD₅₀: 1231 毫克/公斤
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-硝基苄胺 p-nitrobenzylamine 7409-30-5 C7H8N2O2 152.153 4-硝基苄基碘化物 4-nitrobenzyl iodide 3145-86-6 C7H6INO2 263.035 1-(氯甲基)-4-硝基苯 4-nitrobenzyl chloride 100-14-1 C7H6ClNO2 171.583 对硝基苯甲醛 4-nitrobenzaldehdye 555-16-8 C7H5NO3 151.122 对硝基苯甲醇 4-Nitrobenzyl alcohol 619-73-8 C7H7NO3 153.137 对硝基溴化苄 1-bromomethyl-4-nitro-benzene 100-11-8 C7H6BrNO2 216.034 对硝基苯乙醇 2-(4-nitrophenyl)ethanol 100-27-6 C8H9NO3 167.164 1-(重氮基甲基)-4-硝基苯 (4-nitrophenyl)diazomethane 19479-80-2 C7H5N3O2 163.136 alpha,alpha-二溴-4-硝基甲苯 1-dibromomethyl-4-nitro-benzene 619-75-0 C7H5Br2NO2 294.93 4-硝基苯乙醛 p-nitrophenylacetaldehyde 1460-05-5 C8H7NO3 165.148 —— trans-4,4'-dinitrostilbene 736-31-2 C14H10N2O4 270.244 4-硝基硫氰酸苄酯 4-Nitro-benzylthiocyanat 13287-49-5 C8H6N2O2S 194.214 1-硝基-4-苯基乙炔苯 4-(phenylethynyl)nitrobenzene 1942-30-9 C14H9NO2 223.231 1-硝基-4-[2-(4-硝基苯基)乙炔基]苯 1,2-bis(4-nitrophenyl)acetylene 2735-14-0 C14H8N2O4 268.229 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 对硝基甲苯 1-methyl-4-nitrosobenzene 623-11-0 C7H7NO 121.139 N-(对甲苯基)羟胺 N-(4-methylphenyl)hydroxylamine 623-10-9 C7H9NO 123.155 2-氨基-4-硝基甲苯 2-methyl-5-nitroaniline 99-55-8 C7H8N2O2 152.153 对硝基苯乙酸 4-nitrobenzeneacetic acid 104-03-0 C8H7NO4 181.148 2-碘-4-硝基甲苯 2-iodo-1-methyl-4-nitrobenzene 7745-92-8 C7H6INO2 263.035 (4-硝基苄基)膦酸 4-nitrobenzylphosphonic acid 1205-62-5 C7H8NO5P 217.118 2-羟基-2-(4-硝基苯基)乙腈 p-nitromandelonitrile 121986-07-0 C8H6N2O3 178.147 1-硝基-4-(苯氧基甲基)苯 4-nitrobenzyl phenyl ether 3048-12-2 C13H11NO3 229.235 —— 4-nitrobenzylphenyl sulfide 7703-38-0 C13H11NO2S 245.302 3-碘-4-硝基甲苯 3-iodo-4-nitrotoluene 52488-29-6 C7H6INO2 263.035 4-硝基碘苯 4-Iodonitrobenzene 636-98-6 C6H4INO2 249.008 对硝基苯酚 4-nitro-phenol 100-02-7 C6H5NO3 139.111 1-溴-4-硝基苯 4-Bromonitrobenzene 586-78-7 C6H4BrNO2 202.007 1-(4-甲氧基苯基)-2-(4-硝基苯基)-乙炔 1-[2-(4-methoxyphenyl)ethynyl]-4-nitrobenzene 39082-40-1 C15H11NO3 253.257 对硝基氯苯 4-chlorobenzonitrile 100-00-5 C6H4ClNO2 157.556 间硝基苯甲醛 3-nitro-benzaldehyde 99-61-6 C7H5NO3 151.122 (4-硝基苯基)苯甲酸甲酯 4-nitrobenzyl benzoate 4457-41-4 C14H11NO4 257.246 alpha-苯基-4-硝基苯乙醇 2-(p-nitrophenyl)-1-phenylethanol 20273-74-9 C14H13NO3 243.262 - 1
- 2
- 3
- 4
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-硝基苄胺 p-nitrobenzylamine 7409-30-5 C7H8N2O2 152.153 4-硝基苄基碘化物 4-nitrobenzyl iodide 3145-86-6 C7H6INO2 263.035 1-(氟甲基)-4-硝基苯 4-nitrobenzyl fluoride 500-11-8 C7H6FNO2 155.129 对硝基苯甲腈 4-nitrobenzonitrile 619-72-7 C7H4N2O2 148.121 4-硝基苯乙烯 4-nitrostyrene 100-13-0 C8H7NO2 149.149 1-(氯甲基)-4-硝基苯 4-nitrobenzyl chloride 100-14-1 C7H6ClNO2 171.583 对硝基苯甲醛 4-nitrobenzaldehdye 555-16-8 C7H5NO3 151.122 4-硝基-苯甲硫醇 (p-nitrophenyl)methanethiol 26798-33-4 C7H7NO2S 169.204 对硝基苯甲醇 4-Nitrobenzyl alcohol 619-73-8 C7H7NO3 153.137 对硝基溴化苄 1-bromomethyl-4-nitro-benzene 100-11-8 C7H6BrNO2 216.034 4-二氟甲基硝基苯 4-(difluoromethyl)nitrobenzene 29848-57-5 C7H5F2NO2 173.119 —— 4-nitrobenzaldehyde oxime 1129-37-9 C7H6N2O3 166.136 1-(二氯甲基)-4-硝基苯 1-dichloromethyl-4-nitrobenzene 619-78-3 C7H5Cl2NO2 206.028 对硝基苯乙醇 2-(4-nitrophenyl)ethanol 100-27-6 C8H9NO3 167.164 对硝基苯乙腈 4-Nitrophenylacetonitrile 555-21-5 C8H6N2O2 162.148 alpha,alpha-二溴-4-硝基甲苯 1-dibromomethyl-4-nitro-benzene 619-75-0 C7H5Br2NO2 294.93 硝基二苯基甲烷 4-benzyl-1-nitrobenzene 1817-77-2 C13H11NO2 213.236 —— p-nitrobenzyl hidroperoxide 74127-43-8 C7H7NO4 169.137 4-硝基苯乙醛 p-nitrophenylacetaldehyde 1460-05-5 C8H7NO3 165.148 —— 4,4'-dinitro stilbene 619-93-2 C14H10N2O4 270.244 —— trans-4,4'-dinitrostilbene 736-31-2 C14H10N2O4 270.244 1-硝基-4-((E)-苯乙烯基)-苯 trans-4-nitrostilbene 1694-20-8 C14H11NO2 225.247 4-硝基均二苯乙烯 4-nitrostilbene 4003-94-5 C14H11NO2 225.247 顺-4-硝基二苯乙烯 (Z)-1-nitro-4-(2-phenylethenyl)-benzene 6624-53-9 C14H11NO2 225.247 4,4-二硝基联苄 1,2-bis(4-nitrophenyl)ethane 736-30-1 C14H12N2O4 272.26 1-硝基-4-(三氯甲基)苯 1-nitro-4-(trichloromethyl)benzene 3284-64-8 C7H4Cl3NO2 240.473 苯,1-(叠氮甲基)-4-硝基- p-nitrobenzyl azide 17271-88-4 C7H6N4O2 178.15 1-硝基-4-(三溴甲基)苯 4-Nitrobenzotribromide 14505-17-0 C7H4Br3NO2 373.826 硝基苯 nitrobenzene 98-95-3 C6H5NO2 123.111 对硝基甲苯 1-methyl-4-nitrosobenzene 623-11-0 C7H7NO 121.139 —— bis(4-nitrobenzyl) sulfide 1835-71-8 C14H12N2O4S 304.326 N-(对甲苯基)羟胺 N-(4-methylphenyl)hydroxylamine 623-10-9 C7H9NO 123.155 1-硝基-4-(硝基甲基)苯 1-nitro-4-(nitromethyl)benzene 1610-26-0 C7H6N2O4 182.136 1,4-二(4'-硝基苯乙烯基)苯 1,4-bis((E)-4-nitrostyryl)benzene 51042-58-1 C22H16N2O4 372.38 —— (E)-(N,N-dimethylamino)-4-nitrostyrene 136795-65-8 C10H12N2O2 192.217 —— 1-dimethylamino-2-p-nitrophenyl-ethylene 20973-68-6 C10H12N2O2 192.217 对硝基苯甲酰胺 4-nitrobenzamide 619-80-7 C7H6N2O3 166.136 1-(4-硝基苯基)乙醇 1-[4-nitrophenyl]-1-ethanol 6531-13-1 C8H9NO3 167.164 对硝基苯乙酸 4-nitrobenzeneacetic acid 104-03-0 C8H7NO4 181.148 对硝基苯甲酸 4-nitro-benzoic acid 62-23-7 C7H5NO4 167.121 4-硝基苯甲酰氯 4-nitro-benzoyl chloride 122-04-3 C7H4ClNO3 185.567 —— 1-(diphenylmethyl)-4-nitrobenzene 2945-12-2 C19H15NO2 289.334 —— α-bromo-α-fluoro-4-nitrotoluene 51229-65-3 C7H5BrFNO2 234.025 1-(二甲氧基甲基)-4-硝基苯 4-nitrobenzaldehyde dimethyl acetal 881-67-4 C9H11NO4 197.191 2-(4-硝基苯基)-1,3-丙烷二醇 2-(4-nitrophenyl)propane-1,3-diol 91748-03-7 C9H11NO4 197.191 乙酸对硝基苯甲酯 4-nitrobenzyl acetate 619-90-9 C9H9NO4 195.175 —— 4-nitrobenzyl nitrate 15539-77-2 C7H6N2O5 198.135 (4-硝基苄基亚基)苯胺 p-nitrobenzylideneaniline 785-80-8 C13H10N2O2 226.235 —— 4-Dimethylamino-1- -butadien-(1.3) 5159-57-9 C12H14N2O2 218.255 —— N-(4-nitrobenzyl)aniline 10359-18-9 C13H12N2O2 228.25 —— (E)-4-fluoro-4’-nitrostilbene —— C14H10FNO2 243.237 1-氯-4-[(E)-2-(4-硝基苯基)乙烯基]苯 (E)-4-chloro-4'-nitrostilbene 14064-55-2 C14H10ClNO2 259.692 —— N-[1-(4-methyl-phenyl)-methylidene]-N-[4-nitrophenyl]amine 33442-37-4 C14H12N2O2 240.261 2-氯-4-硝基甲苯 2-chloro-4-nitrotoluene 121-86-8 C7H6ClNO2 171.583 对二硝基苯 para-dinitrobenzene 100-25-4 C6H4N2O4 168.109 —— 4-hydroxy-4'-nitrostilbene 19221-08-0 C14H11NO3 241.246 2-氟-4-硝基甲苯 2-fluoro-4-nitrotoluene 1427-07-2 C7H6FNO2 155.129 —— 4-nitro-benzaldehyde phenylhydrazone 2829-27-8 C13H11N3O2 241.249 2-溴-4-硝基甲苯 2-bromo-4-nitrotoluene 7745-93-9 C7H6BrNO2 216.034 2-碘-4-硝基甲苯 2-iodo-1-methyl-4-nitrobenzene 7745-92-8 C7H6INO2 263.035 4-二甲氨基-4'-亚硝基苯乙烯 N,N-dimethyl-4-[(E)-2-(4-nitrophenyl)vinyl]aniline 2844-15-7 C16H16N2O2 268.315 4-二甲基氨基-4'-硝基茋 4-dimethylamino-4'-nitrostilbene 4584-57-0 C16H16N2O2 268.315 对硝基苯甲酸甲酯 4-nitrobenzoic acid methyl ester 619-50-1 C8H7NO4 181.148 2-(4-硝基苯基)丙-2-烯-1-醇 2-(4-nitrophenyl)allyl alcohol 15121-86-5 C9H9NO3 179.175 —— (R)-(-)-1-(4-nitrophenyl)-1,2-ethanediol 77977-74-3 C8H9NO4 183.164 —— (S)-(+)-1-(4-nitrophenyl)-1,2-ethanediol 77977-75-4 C8H9NO4 183.164 —— N-[(4-nitrophenyl)methyl]methanesulphonamide 81880-94-6 C8H10N2O4S 230.244 —— 4-nitro-dithiobenzoic acid methyl ester 58863-41-5 C8H7NO2S2 213.281 1-硝基-4-(苯氧基甲基)苯 4-nitrobenzyl phenyl ether 3048-12-2 C13H11NO3 229.235 —— 4-nitrobenzylphenyl sulfide 7703-38-0 C13H11NO2S 245.302 —— 2-(hydroxymethyl)-2-(4-nitrophenyl)-1,3-propanediol 91748-04-8 C10H13NO5 227.217 3-碘-4-硝基甲苯 3-iodo-4-nitrotoluene 52488-29-6 C7H6INO2 263.035 1-(二乙氧基甲基)-4-硝基苯 p-nitrobenzaldehyde diethyl acetal 2403-62-5 C11H15NO4 225.244 1,3-二氯-2-甲基-5-硝基苯 1,3-dichloro-2-methyl-5-nitrobenzene 7149-69-1 C7H5Cl2NO2 206.028 1-[(E)-2-(4-甲氧基苯基)乙烯基]-4-硝基苯 (E)-1-methoxy-4-(4-nitrostyryl)benzene 4648-33-3 C15H13NO3 255.273 —— 1-methoxy-4-[2-(4-nitrophenyl)ethenyl]benzene —— C15H13NO3 255.273 —— (Z)-1-methoxy-4-[2-(4-nitrophenyl)ethenyl]benzene 4648-14-0 C15H13NO3 255.273 1-溴-4-硝基苯 4-Bromonitrobenzene 586-78-7 C6H4BrNO2 202.007 —— (E)-1-nitro-4-(4-(trifluoromethyl)styryl)benzene 74518-96-0 C15H10F3NO2 293.245 1,3-二溴-2-甲基-5-硝基苯 1,3-dibromo-2-methyl-5-nitrobenzene 101581-06-0 C7H5Br2NO2 294.93 对硝基苯甲酸乙酯 ethyl 4-nitrobenzoate 99-77-4 C9H9NO4 195.175 N,N-二甲基-4-硝基苯甲酰胺 N,N-dimethyl-4-nitrobenzamide 7291-01-2 C9H10N2O3 194.19 —— 1-(4-nitrobenzyl)naphthalene 3137-11-9 C17H13NO2 263.296 (2-甲基-5-硝基-苯基)-甲醇 (2-methyl-5-nitrophenyl)methanol 22474-47-1 C8H9NO3 167.164 1-甲基-4-(4-硝基苯)哌嗪 1-methyl-4-(4-nitrobenzyl)piperazine 70261-81-3 C12H17N3O2 235.286 2,4-二硝基甲苯 2,4-DNt 121-14-2 C7H6N2O4 182.136 —— 3-cyclohexyl-2,3,6,7-tetrahydro-2-(4-nitrophenyl)-cyclopenta[e][1,3]oxazin-4(5H)-one 42974-61-8 C13H16N2O2 232.282 —— 2-methyl-5-nitro-benzylamine 100708-81-4 C8H10N2O2 166.18 —— bis(p-nitrobenzyl) sulfone 117095-76-8 C14H12N2O6S 336.325 (4-硝基苯基)苯甲酸甲酯 4-nitrobenzyl benzoate 4457-41-4 C14H11NO4 257.246 —— N-(4-nitrobenzyl)ethanesulfonamide 1224932-62-0 C9H12N2O4S 244.271 —— 4-(4-nitrostyryl)triphenylamine 142677-07-4 C26H20N2O2 392.457 4-(4-硝基苯乙烯基)三苯胺 4-nitro-4'-(N,N-diphenylamino)stilbene 142677-07-4 C26H20N2O2 392.457 —— 1,4-dimethyl-2-(4-nitrobenzyl)benzene 85716-73-0 C15H15NO2 241.29 2-(氯甲基)-1-甲基-4-硝基苯 2-(chloromethyl)-4-nitrotoluene 58966-24-8 C8H8ClNO2 185.61 4-(4-硝基苄基)吗啡啉 4-(4-nitrobenzyl)morpholine 6425-46-3 C11H14N2O3 222.244 —— 1-(diisopropoxymethyl)-4-nitrobenzene 54149-72-3 C13H19NO4 253.298 —— (p-Nitrobenzyl)trifluoracetat 405-84-5 C9H6F3NO4 249.146 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:4-硝基甲苯 在 N-溴代丁二酰亚胺(NBS) 、 potassium carbonate 、 过氧化苯甲酰 作用下, 以 四氯化碳 、 水 、 苯 为溶剂, 反应 8.25h, 生成 4-硝基苯乙烯参考文献:名称:Phukan; Jagtap; Sudalai, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2000, vol. 39, # 12, p. 950 - 953摘要:DOI:
-
作为产物:描述:5-甲基-2-硝基苯甲酸 在 copper(I) oxide 作用下, 反应 24.0h, 生成 4-硝基甲苯参考文献:名称:Recyclable nanoscale copper(i) catalysts in ionic liquid media for selective decarboxylative C–C bond cleavage摘要:本文报道了在磷鏻离子液体中合成分散良好的5.5 nm至8.0 nm的Cu2O纳米粒子(Cu2O-NPs),并将其应用于作为模型底物的2-硝基苯甲酸及其衍生物的无配体、无添加剂的平滑脱羧反应中,成为首个可循环使用的有效催化体系。该反应使用低剂量的催化剂,并在连续十次循环实验中实现了定量产率。此外,该体系对电子贫乏的2-硝基苯甲酸具有高度选择性。DOI:10.1039/c2cy20760e
-
作为试剂:描述:2,2,6,6-四甲基哌啶氧化物 、 4-甲苯硫酚 在 tris(4,4'-dimethyl-2,2'-bipyridine)ruthenium(II) hexafluorophosphate 、 4-硝基甲苯 、 水 、 N,N-二异丙基乙胺 作用下, 以 二甲基亚砜 为溶剂, 反应 10.0h, 以68%的产率得到2,2,6,6-tetramethyl-1-((p-tolylthio)oxy)piperidine参考文献:名称:硝基芳烃和苯硫酚光催化合成亚磺酰胺和亚砜摘要:我们提出了一种高效且通用的可见光驱动方法,用于合成亚磺酰胺和亚砜,使用硝基芳烃作为氮源,苯硫酚作为硫源。通过改变光催化剂的种类和反应混合物中苯硫酚的含量,实现了两种反应途径的转换。该反应在温和的条件下进行,具有良好的官能团耐受性,易于放大。DOI:10.1021/acs.orglett.2c01824
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Iron-catalyzed thioesterification of methylarenes with thiols in water作者:Liang Wang、Jing Cao、Qun Chen、Ming-yang HeDOI:10.1016/j.tetlet.2014.10.155日期:2014.12An iron-catalyzed coupling reaction of methylarenes with thiols leading to thioesters has been developed. The reactions were carried out in water with tert-butyl hydroperoxide (TBHP) as the oxidant and polyoxyethanyl α-tocopheryl sebacate (PTS) as the surfactant. The reaction medium is compatible with a series of functional groups and can be reused.
-
[EN] POLYCONJUGATES FOR DELIVERY OF RNAI TRIGGERS TO TUMOR CELLS IN VIVO<br/>[FR] POLYCONJUGUÉS POUR L'ADMINISTRATION DE DÉCLENCHEURS D'ARNI À DES CELLULES TUMORALES IN VIVO申请人:ARROWHEAD RES CORP公开号:WO2015021092A1公开(公告)日:2015-02-12The present invention is directed compositions for delivery of RNA interference (RNAi) triggers to integrin positive tumor cells in vivo. The compositions comprise RGD ligand- targeted amphipathic membrane active polyamines reversibly modified with enzyme cleavable dipeptide-amidobenzyl-carbonate masking agents. Modification masks membrane activity of the polymer while reversibility provides physiological responsiveness. The reversibly modified polyamines (dynamic polyconjugate or conjugate) are further covalently linked to an RNAi trigger.
-
Dual Pharmacophores - PDE4-Muscarinic Antagonistics申请人:Callahan James Francis公开号:US20090203657A1公开(公告)日:2009-08-13The present invention is directed to novel compounds of Formula (I) and pharmaceutically acceptable salts thereof, pharmaceutical compositions and their use as dual chromaphores having inhibitory activity against PDE4 and muscarinic acetylcholine receptors (mAChRs), and thus being useful for treating respiratory diseases.本发明涉及具有式(I)的新化合物及其药用盐,药物组合物及其用作对PDE4和肌胆碱受体(mAChRs)具有抑制活性的双色素,因此可用于治疗呼吸道疾病。
-
[EN] DUAL PHARMACOPHORES - PDE4-MUSCARINIC ANTAGONISTICS<br/>[FR] PHARMACOPHORES DUALS, ANTAGONISTES DES RÉCEPTEURS MUSCARINIQUES ET INHIBITEURS DE L'ACTIVITÉ PDE4申请人:GLAXO GROUP LTD公开号:WO2009100169A1公开(公告)日:2009-08-13The present invention is directed to novel compounds of Formula's (I) - (VI), and pharmaceutically acceptable salts thereof, pharmaceutical compositions and their use in therapy, for example as inhibitors of phosphodiesterase type IV (PDE4) and as antagonists of muscarinic acetylcholine receptors (mAChRs), in the treatment of and/or prophylaxis of respiratory diseases, including inflammatory and/or allergic diseases such as chronic obstructive pulmonary disease (COPD), asthma, rhinitis (e.g. allergic rhinitis), atopic dermatitis or psoriasis.
表征谱图
-
氢谱1HNMR
-
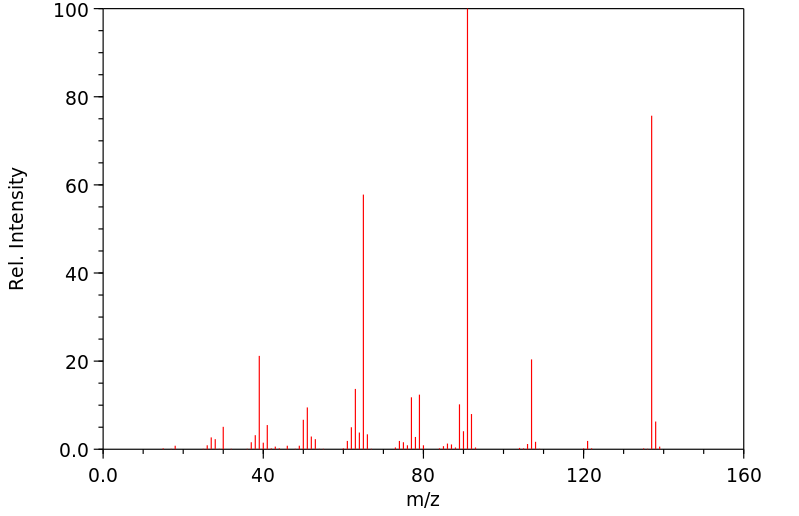
质谱MS
-
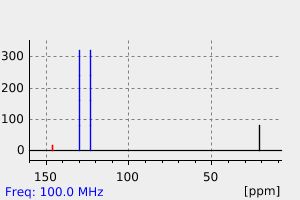
碳谱13CNMR
-
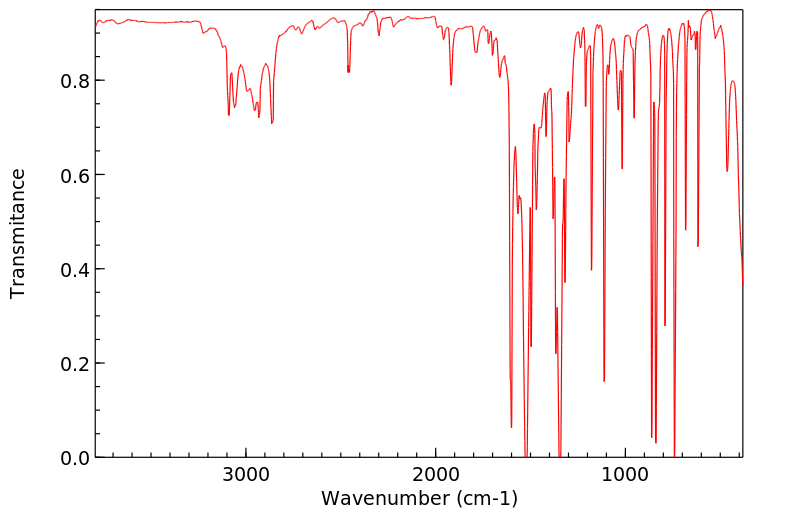
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫