(4-丁氧基苯甲基)三苯基溴化磷 | 146346-92-1
中文名称
(4-丁氧基苯甲基)三苯基溴化磷
中文别名
(4-N-丁氧基苄基)三苯基溴化膦;(4-丁氧基苯甲基)三苯基溴化磷
英文名称
(4-butyloxyphenyl)methyl-triphenylphosphonium bromide
英文别名
(4-butoxybenzyl)-triphenylphosphonium bromide;(4-Butoxybenzyl)triphenylphosphonium bromide;(4-butoxyphenyl)methyl-triphenylphosphanium;bromide
CAS
146346-92-1
化学式
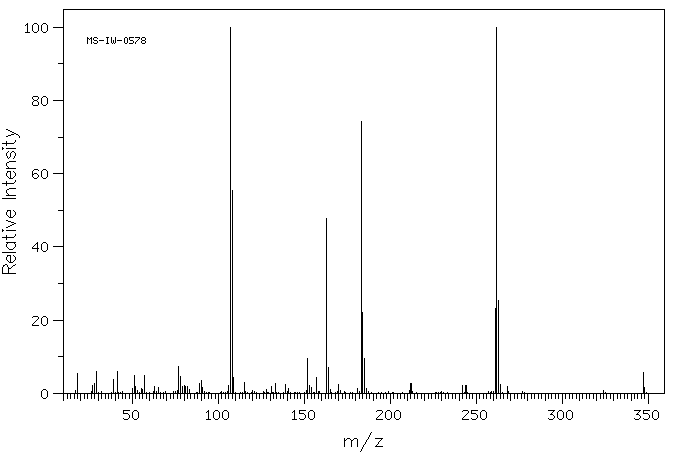
Br*C29H30OP
mdl
——
分子量
505.434
InChiKey
DYBCXFJUMGEGBX-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:195-198 °C(lit.)
计算性质
-
辛醇/水分配系数(LogP):3.36
-
重原子数:32
-
可旋转键数:9
-
环数:4.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
安全说明:S26,S36
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Alkoxylated p-phenylenevinylene oligomers: Synthesis and spectroscopic and electrochemical properties摘要:Twenty-one n-alkoxy substituted phenylenevinylene oligomers were synthesized, varying in size, number and position of the OR groups. IR,MS and solubility data are presented. NMR measurements provided the molecular structure as well as information about conformations and molecular dynamics. UV and of cyclic voltammetric data give correlations of chemical structure (number and position of OR substituents) with separate HOMO and LUMO energies. (C) 1997 Elsevier Science Ltd.DOI:10.1016/s0040-4020(97)00871-5
-
作为产物:描述:参考文献:名称:Alkoxylated p-phenylenevinylene oligomers: Synthesis and spectroscopic and electrochemical properties摘要:Twenty-one n-alkoxy substituted phenylenevinylene oligomers were synthesized, varying in size, number and position of the OR groups. IR,MS and solubility data are presented. NMR measurements provided the molecular structure as well as information about conformations and molecular dynamics. UV and of cyclic voltammetric data give correlations of chemical structure (number and position of OR substituents) with separate HOMO and LUMO energies. (C) 1997 Elsevier Science Ltd.DOI:10.1016/s0040-4020(97)00871-5
文献信息
-
Catalyst for decarbonylation reaction申请人:UBE INDUSTRIES, LTD.公开号:EP0826658A1公开(公告)日:1998-03-04A catalyst composed of an organic phosphorus compound having a trivalent or pentavalent phosphorus atom and at least one carbon-phosphorus bonding or a combination of the organic phosphorus compound and a halogen atom-containing compound is effective for decarbonylation, that is, for releasing carbon monoxide from a compound containing a moiety of -CO-CO-O- in its molecular structure.
-
Quinclidine derivatives as squalene synthase inhibitors申请人:Zeneca Limited公开号:US05691349A1公开(公告)日:1997-11-25Quinuclidine derivatives of formula, and their pharmaceutically acceptable salts, in which: R.sup.1 is hydrogen or hydroxy; R.sup.2 is hydrogen; pr R.sup.1 and R.sup.2 are joined together so that CR.sup.1 -CR.sup.2 is a double bond; X is selected from --CH.sub.2 CH.sub.2 --, --CH.dbd.CH--, --C.dbd.C--, --CH.sub.2 O--, --OCH.sub.2 --, --CH.sub.2 NH--, NHCH.sub.2 --, --CH.sub.2 CO--, --COCH.sub.2 --, --CH.sub.2 S(O).sub.n -- and --S(O).sub.n CH.sub.2 -- wherein n is 0, 1 or 2; and AR is phenyl which may be optionally unsubstituted or substituted by one or more substituents such as halogeno, hydroxy, amino, nitro, cyano, carboxy, carbamoyl, alkyl, alkenyl, alkynyl, alkoxy, alkylamino, dialkylamino, N-alkylcarbamoyl, N,N-di-alkylcarbamoyl, alkoxycarbonyl, alkythio, alkylsulphinyl, alkylsulphonyl, halogeno alkyl, alkanoylamino, alkylenedioxy, alkanoyl and oxime derivatives thereof and O-alkyl ethers of said oximes; provided that when X is selected from --OCH.sub.2 --, --NHCH.sub.2 --, and SCH.sub.2 --, R.sup.1 is not hydroxy; inhibit squalene synthase and are useful in treating diseases or medical conditions in which inhibition of squalene synthase is desirable. The use of such heterocyclic derivatives in treating conditions such as hypercholesterolemia, and atherosclerosis is referred to as well as novel compounds, processes for their preparation and pharmaceutical compositions containing them.喹诺克林衍生物的公式,以及它们的药用可接受盐,其中:R.sup.1是氢或羟基;R.sup.2是氢;或者R.sup.1和R.sup.2连接在一起,使得CR.sup.1-CR.sup.2是一个双键;X选自--CH.sub.2 CH.sub.2 --, --CH.dbd.CH--, --C.dbd.C--, --CH.sub.2 O--, --OCH.sub.2 --, --CH.sub.2 NH--, NHCH.sub.2 --, --CH.sub.2 CO--, --COCH.sub.2 --, --CH.sub.2 S(O).sub.n --和--S(O).sub.n CH.sub.2 --,其中n为0、1或2;AR是苯基,它可以是未取代的,也可以是取代的,取代基可以是卤素、羟基、氨基、硝基、氰基、羧基、氨基甲酰基、烷基、烯基、炔基、烷氧基、烷基氨基、二烷基氨基、N-烷基氨基甲酰基、N,N-二烷基氨基甲酰基、烷氧基羰基、烷基硫、烷基亚磺酰基、烷基亚磺酰基、卤代烷基、烷酰氨基、烷二氧基、烷酰和肟衍生物以及所述肟的O-烷基醚;当X选自--OCH.sub.2 --, --NHCH.sub.2 --和--SCH.sub.2 --时,R.sup.1不是羟基;抑制角鲨烯合酶,在抑制角鲨烯合酶是可取的情况下,用于治疗疾病或医疗状况。此类杂环衍生物在治疗高胆固醇血症和动脉粥样硬化等状况中的应用,以及新的化合物、它们的制备过程和含有它们的药物组合物。
-
Phenylethynyl and styryl derivatives of imidazole and fused ring heterocycles申请人:——公开号:US20020128263A1公开(公告)日:2002-09-12This invention relates to a compound and the use of the compound of the formula 1 wherein R 1 , R 2 , R 3 , R 4 and R 5 are as defined in the description, A signifies —CH═CH— or —C≡C—; and B signifies 2 wherein R 6 to R 26 , X and Y are as defined in the specification or a pharmaceutically acceptable salt thereof, for use in pharmaceutical compositions for the treatment or prevention of mGluR5 receptor mediated disorders.本发明涉及一种化合物及该化合物的使用,其化学式为1,其中R1、R2、R3、R4和R5如描述中所定义,A表示—CH═CH—或—C≡C—;B表示2,其中R6到R26、X和Y如规范中所定义或其药用可接受的盐,用于制备用于治疗或预防mGluR5受体介导的疾病的制药组合物。
-
Cyclohexane compounds used in liquid crystalline mixtures申请人:Hoffmann-La Roche Inc.公开号:US05346647A1公开(公告)日:1994-09-13Compounds of the formula ##STR1## wherein n stands for the number 0 or 1; R.sup.1 denotes a group R.sup.3 or R.sup.3 --A.sup.3 --Z.sup.2 -- and R.sup.2 denotes a group R.sup.4 or R.sup.4 --A.sup.4 --Z.sup.3 --; ring A.sup.1 is unsubstituted or halogen-, cyano- and/or methylsubstituted 1,4-phenylene in which optionally 1 CH group or 2 CH groups is/are replaced by nitrogen; A.sup.3 A.sup.4 and ring A.sup.2 each independently represent unsubstituted or halogen-, cyano- and/or methyl- -substituted 1,4-phenylene in which optionally 1 CH group or 2 CH groups is/are replaced by nitrogen or trans-1,4-cyclohexylene, trans-1,3-dioxane-2,5-diyl, 1-cyano-trans-1,4-cyclohexylene, bicyclo[2.2.2]octane- -1,4-diyl, naphthalene-2,6-diyl, tetralin-2,6-diyl or trans-decalin-2,6- diyl; Z.sup.1, Z.sup.2 and Z.sup.3 each independently are a single covalent bond, --COO--, --OOC--, --CH.sub.2 O--, --OCH.sub.2 --, --CH.sub.2 CH.sub.2 --, --C.vertline.C--, --(CH.sub.2).sub.3 O--, --O(CH.sub.2).sub.3 --, --(CH.sub.2).sub.4 -- or the trans form of --CH.dbd.CH-- CH.sub.2 O-- or --OCH.sub.2 --CH.dbd.CH--; R.sup.3 and R.sup.4 each independently denote halogen, cyano, --NCS, --CF.sub.3, --OCF.sub.3 or alkyl in which optionally one >CH--CH< is replaced by >C.dbd.C< and/or optionally one methylene group or two non- adjacent methylene groups is/are replaced by --O--, --COO-- and/or --OOC-- and/or optionally one methylene group is replaced by --CHX--; and X is halogen, cyano or methyl, as well as liquid crystalline mixtures which contain such compounds and their use for electro-optical purposes.化合物的公式为##STR1##其中n代表数字0或1; R.sup.1表示R.sup.3或R.sup.3 - A.sup.3 - Z.sup.2 -基团,R.sup.2表示R.sup.4或R.sup.4 - A.sup.4 - Z.sup.3 -基团; 环A.sup.1是未取代或卤素,氰基和/或甲基取代的1,4-苯撑基,在其中可以选择将1个CH基团或2个CH基团替换为氮; A.sup.3,A.sup.4和环A.sup.2各自独立地表示未取代或卤素,氰基和/或甲基取代的1,4-苯撑基,在其中可以选择将1个CH基团或2个CH基团替换为氮或反-1,4-环己撑基,反-1,3-二氧杂环戊烷-2,5-二基,1-氰基-反-1,4-环己撑基,双环[2.2.2]辛烷-1,4-二基,萘-2,6-二基,四氢萘-2,6-二基或反-癸烷-2,6-二基; Z.sup.1,Z.sup.2和Z.sup.3各自独立地为单个共价键,-COO-,-OOC-,-CH.sub.2 O-,-OCH.sub.2 -,-CH.sub.2 CH.sub.2 -,-C.vertline.C-,-(CH.sub.2).sub.3 O-,-O(CH.sub.2).sub.3 -,-(CH.sub.2).sub.4-或-CH.dbd.CH-CH.sub.2 O-或-OCH.sub.2-CH.dbd.CH-的反式形式; R.sup.3和R.sup.4各自独立地表示卤素,氰基,-NCS,-CF.sub.3,-OCF.sub.3或烷基,其中可以选择一个>CH-CH<被>C.dbd.C<替换,和/或可以选择一个亚甲基或两个非相邻亚甲基被替换为-O-,-COO-和/或-OOC-,和/或可以选择一个亚甲基被替换为-CHX-; X是卤素,氰基或甲基,以及包含这些化合物的液晶混合物及其用途,用于电光目的。
-
PHOTOACTIVABLE NITROGEN BASES申请人:Dietliker Kurt公开号:US20100105794A1公开(公告)日:2010-04-29Compounds of the Formula (I), (II) and (III) wherein Ar is for example phenylene, biphenylene or naphthylene, all of which are unsubstituted or substituted by C 1 -C 4 -alkyl, C 2 -C 4 -alkenyl, CN, OR 11 , SR 11 , CH2OR 11 , COOR 12 , CONR 12 R 13 or halogen; R 1 , R 2 , R 7 and R 8 independently of one another other are hydrogen or C 1 -C 6 -alkyl; R 3 and R 5 together and R 4 and R 6 together form a C 2 -C 6 -alkylene bridge which is unsubstituted or substituted by one or more C 1 -C 4 -alkyl; R 11 is hydrogen or C 1 -C 6 -alkyl; R 12 and R 13 independently of one another for example are hydrogen, phenyl, C 1 -C 18 -alkyl, C 1 -C 18 -alkyl which is interrupted by one or more O; n is 1-10; X is O, S or NR 10 ; A and A 1 are suitable linking groups; are suitable as photolatent bases.
表征谱图
-
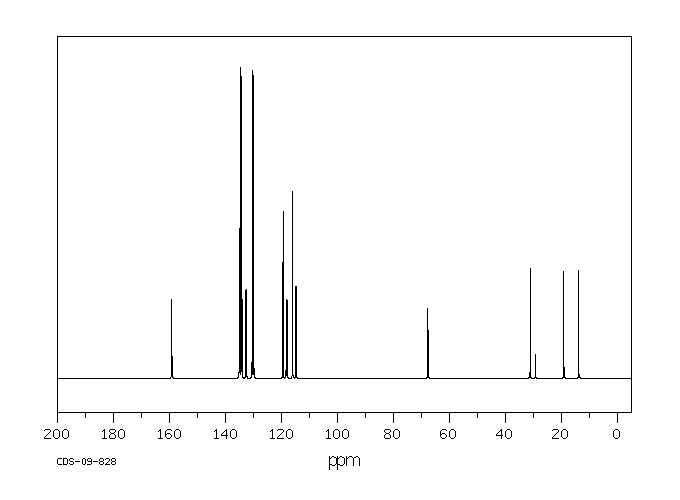
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
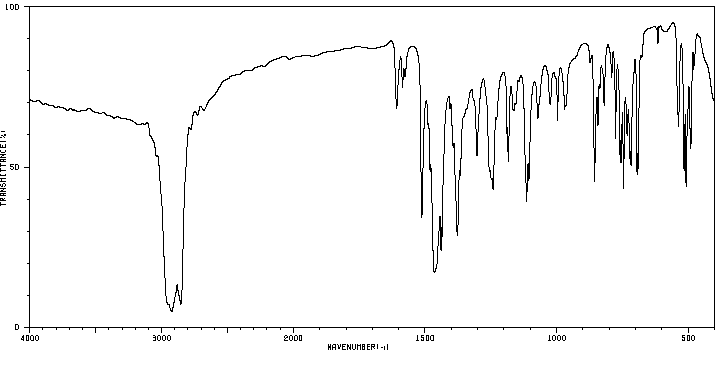
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫