肉桂酸 | 621-82-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:133 °C(lit.)
-
沸点:300 °C(lit.)
-
密度:1.2475
-
闪点:>230 °F
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:2.13
-
物理描述:White crystalline scales, honey floral odour
-
保留指数:1386;1396;1395;1394;1389.1;1394
-
稳定性/保质期:
- 远离氧化物。
- 存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:1
-
海关编码:2916190090
-
危险品运输编号:20kgs
-
RTECS号:GD7850000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存放在密封容器中,并放置在阴凉、通风且干燥的地方,避免阳光直射。储存区域需远离氧化剂。
SDS
| Name: | Cinnamic Acid Material Safety Data Sheet |
| Synonym: | Phenylacrylic acid; 3-Phenylacrylic acid; 3-Phenyl-2-propenoic acid |
| CAS: | 621-82-9 |
Synonym:Phenylacrylic acid; 3-Phenylacrylic acid; 3-Phenyl-2-propenoic acid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 621-82-9 | Cinnamic Acid | 100 % | 210-708-3 |
Risk Phrases: 36/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
Causes gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Reduce airborne dust and prevent scattering by moistening with water. Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Wash hands before eating. Use with adequate ventilation. Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 621-82-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Honey floral odor.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 300 deg C
Freezing/Melting Point: 133 deg C
Autoignition Temperature: Not applicable.
Flash Point: 212 deg F ( 100.00 deg C)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Insoluble in water.
Specific Gravity/Density: Not available.
Molecular Formula: C9H8O2
Molecular Weight: 148.0548
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidants.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 621-82-9: GD7800000 LD50/LC50:
CAS# 621-82-9: Draize test, rabbit, skin: 500 mg/24H Mild; Oral, mouse: LD50 = 5 gm/kg; Oral, rat: LD50 = 2500 mg/kg.
Carcinogenicity:
Cinnamic Acid - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/38 Irritating to eyes and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28 After contact with skin, wash immediately
with...
WGK (Water Danger/Protection)
CAS# 621-82-9: 1
Canada
CAS# 621-82-9 is listed on Canada's DSL List.
CAS# 621-82-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 621-82-9 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
肉桂酸亦称桂皮酸、亚苄基乙酸或3-苯基-2-丙烯酸,是一种不饱和芳香酸。它具有微弱的桂皮香气,并以游离或酯的形式存在于凤仙花、桂皮油与古柯叶中。由于含有双键,肉桂酸有顺式和反式的两种异构体,其中顺式体存在三种同质多晶体。顺式和反式异构体在自然界均有分布,反式存在于苏合香脂、桂皮油、秘鲁香脂、罗勒油及可可叶等精油中;而顺式则主要存在于马六甲良姜油中。市售肉桂酸多为反式。
肉桂酸的相对分子质量为148.17。顺式的别肉桂酸第一种晶型在水中析出后,呈现单斜晶系,无色至白色棱柱状结晶;其相对密度为1.284(4℃),熔点为42℃,沸点分别为265℃(分解)和125℃(2.533×10^3 Pa)。顺式肉桂酸微溶于水,在25℃时溶解度为0.937 g/100 mL,易溶于乙醇、乙醚和乙酸乙酯。
顺式的第二种晶型α-异肉桂酸从石油英中析出后,同样呈现单斜晶系无色至白色棱柱状晶体;其熔点为58℃,沸点265℃。它在乙醇、乙酸、氯仿和丙酮中的溶解度良好,并且易溶于乙醚、丙酮、冰醋酸及油类。
顺式的第三种晶型β-异肉桂酸也呈现单斜晶系无色至白色棱柱状晶体;其熔点为45℃,沸点268.9℃。它在乙醇和氯仿中的溶解度较好,并易溶于乙醚、丙酮、冰醋酸及油类。
反式肉桂酸的溶解性与顺式相似,但相对密度较低(约1.30 g/cm^3),熔点约为45-62℃。它在乙醇和氯仿中的溶解度较好,并易溶于乙醚、丙酮、冰醋酸及油类。
含量分析准确称取预先在盛有硅胶的干燥器中干燥过3小时后的试样500 mg,加入预经用0.1 mol/L氢氧化钠溶液处理的水进行滴定。通过此方法可以测定肉桂酸的含量。
毒性GRAS(一般认为安全)(FEMA)。LD₅₀ 2500 mg/kg(大鼠,经口)
使用限量- FEMA(mg/kg):软饮料31;冷饮40;糖果30;焙烤食品36;胶姆糖10。
- 适度为限(FDA§172.515,2000)
肉桂酸为白色单斜棱晶。微有桂皮香气。易溶于乙醇、甲醇、石油醚、氯仿,亦易溶于苯、乙醚、丙酮、冰醋酸及二硫化碳和油类,而不溶于水。
用途 生产方法以上生产方法均适用于制备肉桂酸。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 肉桂酸甲酯 methyl cinnamate 103-26-4 C10H10O2 162.188 肉桂酸乙酯 ethyl 3-phenyl-2-propenoate 103-36-6 C11H12O2 176.215 肉桂醇 3-Phenylpropenol 104-54-1 C9H10O 134.178 肉桂醛 3-phenyl-propenal 104-55-2 C9H8O 132.162 反式肉桂醛 (E)-3-phenylpropenal 14371-10-9 C9H8O 132.162 肉桂酸烯丙酯 allyl cinnamate 1866-31-5 C12H12O2 188.226 对溴肉桂酸 3-(4-bromophenyl)acrylic acid 1200-07-3 C9H7BrO2 227.057 对羟基肉桂酸 p-Coumaric acid 7400-08-0 C9H8O3 164.161 4-香豆酸 p-Coumaric Acid 501-98-4 C9H8O3 164.161 3-苯基-2-丙烯酸-1-甲乙酯 isopropyl cinnamate 7780-06-5 C12H14O2 190.242 —— tert-butyl cinnamate 14990-09-1 C13H16O2 204.269 肉桂酸椒丁酯 tert-butyl cinnamate 14990-09-1 C13H16O2 204.269 —— (E)-2-Bromo-3-phenyl-acrylic acid 15894-30-1 C9H7BrO2 227.057 (Z)-2-溴-3-苯基丙烯酸 (Z)-2-bromo-3-phenylacrylic acid 15813-24-8 C9H7BrO2 227.057 苄叉丙酮 1-Phenylbut-1-en-3-one 122-57-6 C10H10O 146.189 苄叉丙酮 (E)-benzalacetone 1896-62-4 C10H10O 146.189 肉桂酰胺 cinnamamide 621-79-4 C9H9NO 147.177 肉桂酰氯 cinnamoyl chloride 102-92-1 C9H7ClO 166.607 —— (Z)-3-iodo-3-phenyl-2-propenoic acid 18777-04-3 C9H7IO2 274.058 —— (Z)-3-bromo-3-phenylpropenoic acid 704-78-9 C9H7BrO2 227.057 —— (E)-3-iodo-3-phenyl-2-propenoic acid 18777-04-3 C9H7IO2 274.058 亚苄基丙二酸 2-benzylidenemalonic acid 584-45-2 C10H8O4 192.171 肉桂酸辛酯 cinnamic acid octyl ester 69038-78-4 C17H24O2 260.376 3-(2-氨基苯基)丙烯酸 (E)-3-(2-aminophenyl)-2-propenoic acid 22469-15-4 C9H9NO2 163.176 —— 1,5-diphenyl-1,4-pentadien-3-one 538-58-9 C17H14O 234.298 —— 4-Isopropyl-zimtsaeureethylester 32580-69-1 C14H18O2 218.296 4-(3-羟基丙-1-烯基)苯酚 (E)-4-(3-hydroxyprop-1-enyl)phenol 20649-40-5 C9H10O2 150.177 —— cinnamoyl cyanide 60299-77-6 C10H7NO 157.172 —— N-methylcinnamamide 2757-10-0 C10H11NO 161.203 肉桂酸苄酯 benzy cinnamate 103-41-3 C16H14O2 238.286 TRANS-咖啡酸 caffeic acid 501-16-6 C9H8O4 180.16 肉桂醛肟 cinnamaldoxime 13372-81-1 C9H9NO 147.177 N-羟基-3-苯基-2-丙烯-1-亚胺 cinnamaldehyde-(Z)-oxime 20707-70-4 C9H9NO 147.177 (E)-2-氧代-4-苯基-3-丁烯酸 (E)-2-oxo-4-phenyl-3-butenoic acid 1914-59-6 C10H8O3 176.172 桂醛乙二缩醛 2-((E)-Styryl)-[1,3]dioxolane 5660-60-6 C11H12O2 176.215 3-(4-甲氧基苯基)-2-丙烯酸丙酯 4-methoxycinnamic acid propylester 68141-12-8 C13H16O3 220.268 对甲氧基肉桂酸异丙酯 isopropyl 4-methoxycinnamate 5466-76-2 C13H16O3 220.268 3-溴-3-苯基丙-2-烯醛 3-bromo-3-phenylacrylaldehyde 14804-59-2 C9H7BrO 211.058 (E)-N-甲氧基肉桂酰胺 N-methoxycinnamamide 112404-04-3 C10H11NO2 177.203 2-丙烯酰胺,N,N-二甲基-3-苯基-,(2E)- (E)-N,N-dimethylcinnamamide 17431-39-9 C11H13NO 175.23 —— N,N-dimethyl-3-phenylacrylamide —— C11H13NO 175.23 (E)-肉桂酸苯酯 phenyl cinnamate 25695-77-6 C15H12O2 224.259 —— cinnamoyl azide 26829-64-1 C9H7N3O 173.174 6-苯基-六-3,5-二烯-2-酮 1-phenyl-1,3-hexadien-5-one 4173-44-8 C12H12O 172.227 —— (E)-5-Phenyl-pent-4-ene-2,3-diol 66820-55-1 C11H14O2 178.231 - 1
- 2
- 3
- 4
- 5
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 別桂皮酸 cis-cinnamic acid 102-94-3 C9H8O2 148.161 肉桂酸甲酯 methyl cinnamate 103-26-4 C10H10O2 162.188 —— Methyl cinnamate 103-26-4 C10H10O2 162.188 肉桂酸乙酯 ethyl 3-phenyl-2-propenoate 103-36-6 C11H12O2 176.215 反式-肉桂酸乙酯 ethyl cinnamate 4192-77-2 C11H12O2 176.215 肉桂酸乙烯酯 vinyl cinnamate 3098-92-8 C11H10O2 174.199 肉桂醇 3-Phenylpropenol 104-54-1 C9H10O 134.178 肉桂醛 3-phenyl-propenal 104-55-2 C9H8O 132.162 胺桂皮酸 p-aminocinnamic acid 2393-18-2 C9H9NO2 163.176 肉桂酸烯丙酯 allyl cinnamate 1866-31-5 C12H12O2 188.226 —— p-Iod-zimtsaeure 34633-09-5 C9H7IO2 274.058 4-氨基甲基肉桂酸 4-aminomethyl-cinnamic acid 104566-34-9 C10H11NO2 177.203 —— (E)-allyl cinnamate 56289-56-6 C12H12O2 188.226 对羟基肉桂酸 p-Coumaric acid 7400-08-0 C9H8O3 164.161 桂酸桂酯 3-phenyl-2-propen-1-yl 3-phenylacrylate 122-69-0 C18H16O2 264.324 肉桂酸正丙酯 cinnamic acid propyl ester 7778-83-8 C12H14O2 190.242 —— (E)-cinnamyl cinnamate —— C18H16O2 264.324 —— 2-hydroxyethyl 3-(phenyl)-2-propenoate 146604-63-9 C11H12O3 192.214 2-羟基乙基(2E)-3-苯基丙-2-烯酸酯 2-hydroxyethyl cinnamate 17773-43-2 C11H12O3 192.214 3-苯基-2-丙烯酸-1-甲乙酯 isopropyl cinnamate 7780-06-5 C12H14O2 190.242 —— (E)-isopropyl cinnamate 60512-85-8 C12H14O2 190.242 —— cyanomethyl cinnamate 140935-01-9 C11H9NO2 187.198 —— 2-chloroethyl cinnamate 1115400-44-6 C11H11ClO2 210.66 —— prop-2-yn-1-yl cinnamate 91368-35-3 C12H10O2 186.21 —— (Z)-prop-1-enyl cinnamate 1384266-50-5 C12H12O2 188.226 —— 2-bromoethyl cinnamate 39257-72-2 C11H11BrO2 255.111 —— cinnamyl acetate —— C11H10O3 190.199 桂皮[酸]酐 cinnamic acid anhydride 538-56-7 C18H14O3 278.307 4-氨基肉桂酸甲酯 methyl p-aminocinnamate 65198-02-9 C10H11NO2 177.203 反式-桂皮酸酐 cinnamic anhydride 21947-71-7 C18H14O3 278.307 —— cinnamyl ethyl ether 1476-07-9 C11H14O 162.232 肉桂酸丁酯 n-butyl cinnamate 538-65-8 C13H16O2 204.269 2-氯-3-苯基丙-2-烯酸 (Z)-α-chlorocinnamic acid 705-54-4 C9H7ClO2 182.606 肉桂酸异丁酯 2-propenoic acid, 3-phenyl-, 2-methylpropyl ester 122-67-8 C13H16O2 204.269 —— tert-butyl cinnamate 14990-09-1 C13H16O2 204.269 肉桂酸椒丁酯 tert-butyl cinnamate 14990-09-1 C13H16O2 204.269 —— ((oxybis(ethane-2,1-diyl))bis(oxy))bis(ethane-2,1-diyl) (2E,2′E)-bis(3-phenylacrylate) —— C26H30O7 454.52 —— cinnamoyloxymethyl acetate —— C12H12O4 220.225 —— Bis(4-Hydroxycinnamoyl)methan 32175-76-1 C19H16O4 308.334 苄叉丙酮 1-Phenylbut-1-en-3-one 122-57-6 C10H10O 146.189 —— trimethylsilyl cinnamate 2078-20-8 C12H16O2Si 220.343 肉硅酸戊酯 n-amyl cinnamate 3487-99-8 C14H18O2 218.296 4-苯基-3-丁烯-2-醇 4-phenylbut-3-en-2-ol 17488-65-2 C10H12O 148.205 肉桂酸异戊酯 isoamyl cinnamate 7779-65-9 C14H18O2 218.296 反-肉桂酰胺 cinnamamide 22031-64-7 C9H9NO 147.177 肉桂酰胺 cinnamamide 621-79-4 C9H9NO 147.177 —— 2-oxopropyl cinnamate 70639-28-0 C12H12O3 204.225 —— 4-bromobutyl cinnamate —— C13H15BrO2 283.165 肉桂酰氯 cinnamoyl chloride 102-92-1 C9H7ClO 166.607 3-苯基-2-丙烯酰氯 Cinnamoyl chloride 17082-09-6 C9H7ClO 166.607 —— 4-chlorobutyl cinnamate 63965-44-6 C13H15ClO2 238.714 己基(E)-3-苯基丙-2-烯酸酯 cinnamic acid hexyl ester 3488-00-4 C15H20O2 232.323 —— cinnamoyl fluoride —— C9H7FO 150.152 —— cinnamoyl bromide 105041-05-2 C9H7BrO 211.058 —— (E)-n-heptyl cinnamate 10032-08-3 C16H22O2 246.349 肉桂酸庚酯 n-heptyl cinnamate 10032-08-3 C16H22O2 246.349 肉桂酸辛酯 cinnamic acid octyl ester 69038-78-4 C17H24O2 260.376 —— 3-oxobutyl cinnamate 1417457-41-0 C13H14O3 218.252 肉桂酸辛酯 octyl cinnamate 131751-35-4 C17H24O2 260.376 —— cinnamic acid-(2-methyl-butyl ester) 74559-94-7 C14H18O2 218.296 —— 4-aminocinnamyl alcohol —— C9H11NO 149.192 —— 2-methyl-4-phenylbut-3-en-2-ol —— C11H14O 162.232 —— 1,3-dichloro-2-propyl cinnamate 157140-94-8 C12H12Cl2O2 259.132 (2E,4E)-5-苯基-2,4-戊二烯酸 5-phenylpenta-2,4-dienoic acid 28010-12-0 C11H10O2 174.199 —— mono-cinnamoyl glycerol 68146-51-0 C12H14O4 222.241 —— 1-O-cinnamoyl glycerol 132928-39-3 C12H14O4 222.241 肉桂基氯 cinnamyl chloride 2687-12-9 C9H9Cl 152.623 —— (1E)-1-phenylpent-1-en-3-one 3152-68-9 C11H12O 160.216 (肉)桂腈 cinnamonitrile 4360-47-8 C9H7N 129.161 肉桂腈 cinnamic nitrile 1885-38-7 C9H7N 129.161 —— (carbonic acid ethyl ester)-cinnamic acid-anhydride 108761-28-0 C12H12O4 220.225 —— cinnamoyl cyanide 60299-77-6 C10H7NO 157.172 —— tert-amyl cinnamate 88539-04-2 C14H18O2 218.296 肉桂基溴 cinnamyl bromide 4392-24-9 C9H9Br 197.074 —— 3-phenylacrylic acid hydrazide 3538-69-0 C9H10N2O 162.191 —— 1,3-di-O-Cinnamoyl-glycerol 68146-47-4 C21H20O5 352.387 —— N-methylcinnamamide 2757-10-0 C10H11NO 161.203 —— (E)-cinnamic acid benzyl ester 103-41-3 C16H14O2 238.286 肉桂酸苄酯 benzy cinnamate 103-41-3 C16H14O2 238.286 —— trans-cinnamic acid-(3-chloro-2-hydroxy-propyl ester) 109573-58-2 C12H13ClO3 240.686 反-肉桂酸苯乙酯 2-phenylethyl (E)-cinnamate 63238-64-2 C17H16O2 252.313 肉桂酸苯乙酯 phenethyl cinnamate 103-53-7 C17H16O2 252.313 —— glycidyl cinnamate 19532-86-6 C12H12O3 204.225 (E)-N-羟基-3-苯基丙-2-烯酰胺 N-hydroxycinnamamide 3669-32-7 C9H9NO2 163.176 反-苯乙烯乙酸 (E)-styrylacetic acid 1914-58-5 C10H10O2 162.188 1,4-二苯基1,3-丁二烯 1,4-diphenylbutadiene 886-65-7 C16H14 206.287 3,3-二苯基-2-丙烯酸 3,3-diphenylacrylic acid 606-84-8 C15H12O2 224.259 (E)-4-苯基-3-丁烯腈 (E)-4-phenyl-3-butenenitrile 20068-10-4 C10H9N 143.188 —— 2-formylcinnamic acid 189356-79-4 C10H8O3 176.172 桂酸苯丙酯 3-phenylpropyl cinnamate 122-68-9 C18H18O2 266.34 —— 4-methylbenzyl cinnamate 812655-01-9 C17H16O2 252.313 —— benzylidene pyruvic acid 17451-19-3 C10H8O3 176.172 对硝基肉桂酸 p-nitrocinnamic acid 619-89-6 C9H7NO4 193.159 —— cyclopentyl cinnamate —— C14H16O2 216.28 —— 3-Hydroxy-1-phenyl-hex-1-en 22596-38-9 C12H16O 176.258 —— O-Ethyl 3-phenylthio-2-propenoate 73818-80-1 C11H12OS 192.282 (E)-N-甲氧基肉桂酰胺 N-methoxycinnamamide 112404-04-3 C10H11NO2 177.203 —— cinnamic acid-(1-methyl-heptyl ester) 599178-04-8 C17H24O2 260.376 —— N,N-dimethyl-3-phenylacrylamide —— C11H13NO 175.23 2-丙烯酰胺,N,N-二甲基-3-苯基-,(2E)- (E)-N,N-dimethylcinnamamide 17431-39-9 C11H13NO 175.23 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:参考文献:名称:Jackson; Pasiut, Journal of the American Chemical Society, 1927, vol. 49, p. 2078摘要:DOI:
-
作为产物:参考文献:名称:连续流中连续双催化剂床上的级联好氧选择性氧化摘要:级联反应代表了一种原子经济且节能的技术,可减少化学制造所需的操作次数。生物催化级联在自然界无处不在。然而,控制反应物,中间体和活性位点之间相互作用的顺序仍然是化学催化的挑战。在这里,我们演示了一种通过流化法使用化学催化剂实现高效级联的方法。在连续流动操作下,双床配置中的Pd / SBA-15和Pt / SBA-15非均相催化剂的紧密偶联可提供84%的高单程收率(比批处理操作提高20倍),并且对于>肉桂醇向肉桂酸的级联氧化反应中有14000的周转率,尽管两种催化剂对该反应均没有活性。Pd(第一床)和Pt(第二床)催化剂的合理排序对于促进不希望的加氢和氢解反应的级联氧化至关重要,后者优于逆流床顺序或单个混合的PdPt反应器床。每个床的固有催化性能都保留在最佳的双床配置中,从而能够定量预测其单独氧化行为已建立的反应物/中间体的最终产品收率。使用连续的反应器床进行连续处理可实现采用“简单”催化剂的DOI:10.1021/acscatal.9b00092
-
作为试剂:描述:参考文献:名称:Copper-mediated S–N formation via an oxygen-activated radical process: a new synthesis method for sulfonamides摘要:通过氧活化的自由基过程,已开发出一种铜介导的磺胺酰胺的直接合成。DOI:10.1039/c4cc01353k
文献信息
-
Convenient Preparation of Primary Amides via Activation of Carboxylic Acids with Ethyl Chloroformate and Triethylamine under Mild Conditions作者:Takuya Noguchi、Masahiro Sekine、Yuki Yokoo、Seunghee Jung、Nobuyuki ImaiDOI:10.1246/cl.130096日期:2013.6.5Primary amides were easily prepared in 22–99% yields from the corresponding carboxylic acids 1 or 5 with NH4Cl via activation with ClCO2Et and Et3N. The enantiomers of the corresponding primary ami...
-
Mild reduction of carboxylic acids to alcohols using cyanuric chloride and sodium borohydride作者:Massimo Falorni、Andrea Porcheddu、Maurizio TaddeiDOI:10.1016/s0040-4039(99)00734-0日期:1999.6Several carboxylic acids, including N-Boc, N-Cbz and N-Fmoc amino acids were reduced to the corresponding alcohols by activation of the carboxy function with cyanuric chloride and N-methylmorpholine followed by reduction with aqueous sodium borohydride.
-
一种利用嘧啶类缩合剂点击构建酰胺键的方法及其在酰胺和多肽合成中的应用申请人:兰州大学公开号:CN112851536B公开(公告)日:2022-02-25
-
New and simple synthesis of acid azides, ureas and carbamates from carboxylic acids: application of peptide coupling agents EDC and HBTU作者:Vommina V. Sureshbabu、H. S. Lalithamba、N. Narendra、H. P. HemanthaDOI:10.1039/b920290k日期:——Conversion of carboxylic acids into acid azides using peptide coupling agents, EDC and HBTU is described. The procedure is efficient, practical and applicable to a diverse range of carboxylic acids including N-protected amino acids. Using the same reagents, one-pot synthesis of ureas, dipeptidyl urea esters and carbamates from acids has also been achieved.
-
An efficient mechanochemical synthesis of amides and dipeptides using 2,4,6-trichloro-1,3,5-triazine and PPh<sub>3</sub>作者:Chuthamat Duangkamol、Subin Jaita、Sirilak Wangngae、Wong Phakhodee、Mookda PattarawarapanDOI:10.1039/c5ra10127a日期:——mechanochemical synthesis of amides from carboxylic acids has been developed through an in situ acid activation with 2,4,6-trichloro-1,3,5-triazine and a catalytic amount of PPh3. Under room temperature solvent-drop grinding of the reactants in the presence of an inorganic base, a variety of carboxylic acids including aromatic acids, aliphatic acids, and N-protected α-amino acids undergo amidation to afford
表征谱图
-
氢谱1HNMR
-
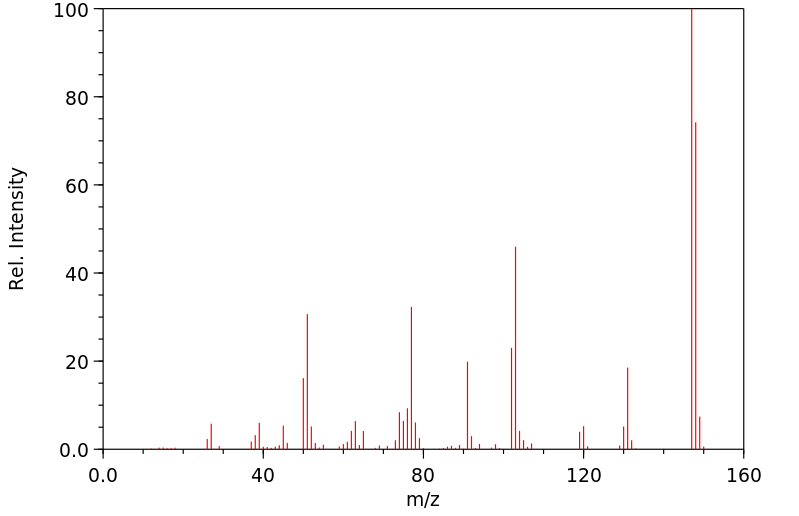
质谱MS
-
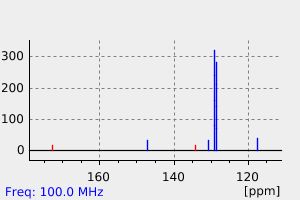
碳谱13CNMR
-
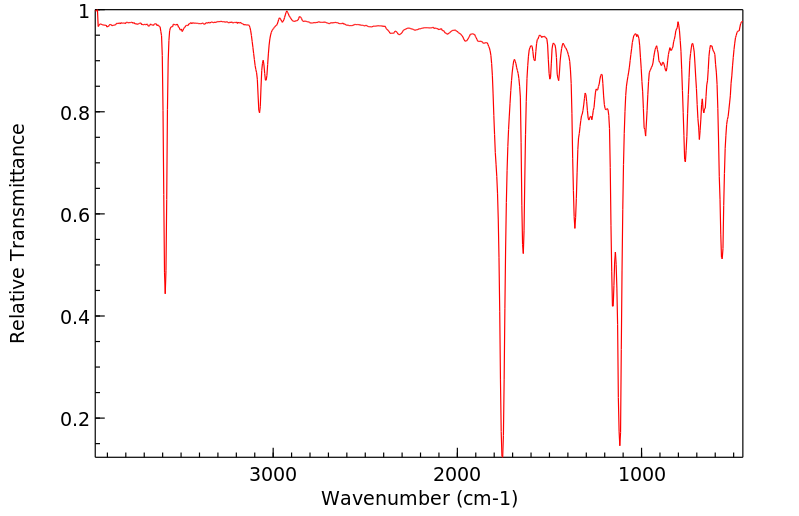
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息