氯 | 7782-50-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-105.5 °C
-
沸点:-34.04 °C
-
密度:1.4 g/cm3(Temp: 20 °C Press: 5210 Torr)
-
物理描述:Chlorine appears as a greenish yellow gas with a pungent suffocating odor. Toxic by inhalation. Slightly soluble in water. Liquefies at -35°C and room pressure. Readily liquefied by pressure applied at room temperature. Density (as a liquid) 13.0 lb / gal. Contact with unconfined liquid can cause frostbite by evaporative cooling. Does not burn but, like oxygen, supports combustion. Long-term inhalation of low concentrations or short-term inhalation of high concentrations has ill effects. Vapors are much heavier than air and tend to settle in low areas. Contact CHEMTREC to activate chlorine response team 800-424-9300. Used to purify water, bleach wood pulp, and to make other chemicals. Rate of onset: Immediate to hours Persistence: Minutes to hours Odor threshold: 3.5 ppm Source/use/other hazard: Cleaner/disinfectant in many industries; water treatment; WWI war gas; irritating corr fumes heavier than air.
-
颜色/状态:Yellowish-green gas
-
气味:Suffocating odor
-
溶解度:1.46 g/100 cc water at 0 °C; 310 cc/100 cc water at 10 °C; 177 cc/100 cc water at 30 °C; 0.57 g/100 cc water at 30 °C
-
蒸汽密度:2.49 (EPA, 1998) (Relative to Air)
-
蒸汽压力:5.83X10+3 mm Hg at 25 °C
-
稳定性/保质期:
-
自燃温度:Not flammable (USCG, 1999)
-
分解:Hazardous decomposition products formed under fire conditions - Nature of decomposition products not known.
-
粘度:0.134 mPa.sec at 20 °C (gas); 0.346 mPa.sec at 20 °C (liquid)
-
腐蚀性:Chlorine will attack some forms of plastics, rubber, and coatings
-
汽化热:20.41 kJ/mol at -34.03 °C; 17.76 kJ/mol at 25 °C
-
表面张力:18.4 dynes/cm at 20 °C in contact with vapor
-
电离电位:11.48 eV
-
气味阈值:Water odor threshold: 0.0020 mg/L. Air odor threshold: 0.31 ppm. Odor Safety Class: C. C= Odor safety factor from 1-26. Less than 50% of distracted persons perceive warning of threshold limit value.
-
折光率:Index of refraction: 1.3834 at 20 °C/589 nm (liquid)
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:2
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
危险等级:2.3
-
危险品标志:T
-
安全说明:S45,S61,S9
-
危险类别码:R50,R36/37/38,R23
-
WGK Germany:2
-
RTECS号:FO2100000
-
海关编码:2801100000
-
危险类别:2.3
-
危险标志:GHS03,GHS04,GHS06,GHS09
-
危险品运输编号:UN 1017 2.3
-
危险性描述:H270,H280,H315,H319,H331,H335,H400
-
危险性防范说明:P220,P244,P261,P304 + P340 + P312,P403 + P233,P410 + P403
制备方法与用途
- 大规模生产氯气的方法
- 1. 通过电解饱和食盐水获得工业液氯。
- 2. 利用漂白粉与盐酸反应制备大量氯气,或利用启普发生器制取少量氯气并提纯。
<dt>净化过程</dt>
<dd>高纯度<a href=https://www.molaid.com/MS_36390 target="_blank">氯</a>气可通过干燥、吸附和冷凝等步骤获得。</dd>
<dt>仪器设备</dt>
<dd>包括D2SO4储器、连接管、盛有NaCl的瓶、DCl接受器及其他辅助设备。</dd>
</dl>
<b class="ip_info4_code">应用领域</b>
<dl class="ip_deslist">
<dt>高新技术领域</dt>
<dd>主要用于大规模集成电路、光纤和高温超导等领域。</dd>
<dt><a href=https://www.molaid.com/MS_34644 target="_blank">水</a>处理与环保</dt>
<dd><a href=https://www.molaid.com/MS_36390 target="_blank">氯</a>作为强氧化剂,能不同程度地氧化冷却<a href=https://www.molaid.com/MS_34644 target="_blank">水</a>中的有机物,并可能生成有毒的<a href=https://www.molaid.com/MS_36390 target="_blank">氯</a>代烃。需配合使用非氧化型杀菌剂和黏泥剥离剂。</dd>
<dt>工业应用</dt>
<dd>1. 纺织品和纸浆漂白。
2. 冶<a href=https://www.molaid.com/MS_5552 target="_blank">金</a>工业中用于生产<a href=https://www.molaid.com/MS_5552 target="_blank">金</a>属<a href=https://www.molaid.com/MS_49916 target="_blank">钛</a>、<a href=https://www.molaid.com/MS_4022 target="_blank">镁</a>等。
3. <a href=https://www.molaid.com/fenzi/4464 target="_blank">化学</a>工业中用于制造<a href=https://www.molaid.com/MS_17977305 target="_blank">次氯酸钠</a>、<a href=https://www.molaid.com/MS_80184 target="_blank">三氯化铝</a>等<a href=https://www.molaid.com/fenzi/4155 target="_blank">无机化工</a>产品及有机<a href=https://www.molaid.com/MS_36390 target="_blank">氯</a>化物,如<a href=https://www.molaid.com/MS_36091 target="_blank">氯乙酸</a>、<a href=https://www.molaid.com/MS_29992 target="_blank">环氧氯丙烷</a>等。
4. 塑料和<a href=https://www.molaid.com/fenzi/4873 target="_blank">增塑剂</a>的生产。
5. 合成洗涤剂原料如烷基<a href=https://www.molaid.com/MS_19374 target="_blank">磺酸钠</a>和烷基<a href=https://www.molaid.com/MS_10857 target="_blank">苯磺酸钠</a>。
6. 农药工业中用作高效<a href=https://www.molaid.com/fenzi/4258 target="_blank">杀虫剂</a>、杀菌剂及<a href=https://www.molaid.com/fenzi/4260 target="_blank">除草剂</a>的原料。
7. 自来<a href=https://www.molaid.com/MS_34644 target="_blank">水</a>消毒与净化。
<dt>半导体行业</dt>
<dd>作为气体蚀刻剂,尤其可与<a href=https://www.molaid.com/MS_80200 target="_blank">三氯化硼</a>混合用于铝的蚀刻。也可应用于晶体生长和热氧化等工艺。</dd>
<dt>其他应用</dt>
<dd>如生产聚<a href=https://www.molaid.com/MS_36132 target="_blank">氯乙烯</a>及制造各种含<a href=https://www.molaid.com/MS_36390 target="_blank">氯</a>化合物、<a href=https://www.molaid.com/MS_23678 target="_blank">盐酸</a>等。</dd>
</dl>
注:原文中提到的制备过程较为复杂且涉及专业设备,简化整理以易于理解。具体操作时请参照标准实验方法与安全规范。
上下游信息
反应信息
-
作为反应物:参考文献:名称:Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Cl: MVol., 98, page 265 - 268摘要:DOI:
-
作为产物:参考文献:名称:时间分辨LMR研究ClNO在基态(2 P )和激发态(2 P )自旋轨道状态下的反应摘要:Nd:YAG激光的三次谐波(354 nm)和三次谐波(266 nm)对ClNO的光解作用已导致Cl精细结构能级的总体反转。Cl(2 P )+ ClNO→Cl 2 + NO的速率常数已确定为(7.7±1.7)×10 -11 cm 3 / s,并且对Cl 2(354 nm),S 2 Cl 2进行了光解。(266 nm)和ICl(530 nm)作为Cl(2 P )的来源。氯(的反应2 P )已被证明是比氯(低得多2 P )。Cl(2 P)已经确定为等于(1.8±0.4)×10 -11 cm 3 / s,ICl(530 nm)和ClNO(354; 266 nm)的光解用作Cl(2 P )的来源。所有结果均在298±5 K的温度下获得。DOI:10.1016/0301-0104(87)80071-x
-
作为试剂:参考文献:名称:用于有机化学和药物化学的 2- 和 3-氟环丁烷结构单元摘要:公开了2-和3-单氟化环丁烷结构单元的多克合成。在这两种情况下,氟环丁烷羧酸是用于获得所有其他衍生物(例如胺、醇、溴化物、硫醇、磺酰氯等)的关键中间体。对于这两种目标化学型,相应羧酸的非对映纯顺式和反式异构体以及通过非对映异构体分离得到胺。还通过模型衍生物的pKa和 Log P测量来表征2-和 3- 氟环丁基取代基。DOI:10.1002/ejoc.202400493
文献信息
-
A European Perspective on Depression in the Community: The DEPRES Study作者:Saena Arbabzadeh-Bouchez、Andre Tylee、Jean-Pierre LépineDOI:10.1017/s1092852900017430日期:2002.2
ABSTRACT Depression is one of the most prevalent disorders in the general population, causing personal and social disability and impairment. Major studies assessing the diagnosis and management of depression have shown that it is often underdiagnosed and undertreated. A pan-European study aimed at assessing the extent and consequences of depression in six different countries is reported in this article. Different types of depressive profiles are analyzed and their respective management has been compared. The importance of improving diagnosis and treatment of depression is underlined. Appropriate management of depression depends on the recognition of depressive symptoms by patients, their possibility of seeking care, and the ability of the primary care physician to recognize the disorder and prescribe the appropriate medicines. Improvement in all of these fields is necessary.
摘要抑郁症是普通人群中最常见的疾病之一,会造成个人和社会的残疾和损伤。对抑郁症的诊断和管理进行评估的主要研究表明,抑郁症往往诊断不足、治疗不力。本文报告了一项泛欧研究,旨在评估六个不同国家抑郁症的程度和后果。研究分析了不同类型的抑郁症,并对其各自的治疗方法进行了比较。文章强调了改进抑郁症诊断和治疗的重要性。抑郁症的适当治疗取决于患者对抑郁症状的认识、他们寻求治疗的可能性以及初级保健医生识别疾病和开具适当药物的能力。所有这些领域都需要改进。 -
Phosphorus–fluorine chemistry. Part XXI. Pentafluorophenylfluorophosphines and pentafluorophenylfluorophosphoranes作者:M. Fild、R. SchmutzlerDOI:10.1039/j19690000840日期:——The preparation of the fluorophosphines, (C6F5)nPF3–n, and of the fluorophosphoranes, (C6F5)nPF5–n, as well as of the related oxygen species, (C6F5)nP(:O)F3–n(n= 1, 2) is described. 19F and 31P N.m.r. data for the new compounds are reported and discussed.
-
Detection of coronary stenoses by stress echocardiography using a previously implanted pacemaker for ventricular pacing: Preliminary report of a new method作者:Daniel Benchimol、Marc Mazanof、HÉLÈNe Benchimol、Virginie Bernard、Thierry Couffinhal、Raymond Roudaut、BÉNÉDicte Dubroca、Jean François Dartigues、Jacques Bonnet、Xaver PilloisDOI:10.1002/clc.4960231111日期:2000.11infarction. HYPOTHESIS To detect significant coronary stenosis in patients with previously implanted pacemakers, we tested a new stress echocardiography method using incremental ventricular pacing by already implanted pacemakers. METHODS We studied prospectively 25 consecutive patients who underwent stress echocardiography with increasing ventricular pacing up to either 85% of the age-predicted maximal背景技术具有起搏器的患者的数量一直在增加,并且其中许多患者将出现胸痛或暗示心绞痛的症状。使用起搏器的患者很难通过无创方法检测和评估心肌缺血和冠状动脉疾病。通常,就缺血甚至急性心肌梗塞而言,静止和运动期间的心电图(ECG)都很难分析。假设为了检测先前植入起搏器的患者的严重冠状动脉狭窄,我们测试了一种新的应力超声心动图方法,该方法采用已经植入的起搏器进行增量心室起搏。方法我们前瞻性地研究了连续25例接受压力超声心动图检查的患者,这些患者的心室起搏增加至年龄预测的最大心率或胸痛的85%。阳性试验是由新的运动功能减退或至少在两个相邻地区壁运动的先前变化加重定义的。所有患者均进行了冠状动脉造影,以确定冠状动脉狭窄的存在和严重程度。结果在25项测试中,有11项(44%)因胸痛而停止使用。中度不适感为1(4%),血压下降为1(4%),其余12位患者(48%)的测试中达到了目标起搏率。没有并发症。13名患
-
Photoreduction of Pt(IV) Halo-Hydroxo Complexes: Possible Hypohalous Acid Elimination作者:Lasantha A. Wickramasinghe、Paul R. SharpDOI:10.1021/ic402358s日期:2014.2.3detected in photolyzed benzene solutions. Photolysis of 3 or 6 in the presence of 2,3-dimethyl-2-butene (TME) yields the chlorohydrin (2-chloro-2,3-dimethyl-3-butanol), 3-chloro-2,3-dimethyl-1-butene, and acetone, all expected products from HOCl trapping, but additional oxidation products are also observed. Photolysis of mixed chloro-bromo complex 7 with TME yields the bromohydrin (2-bromo-2,3-dimethyl-3-butanol)将浓缩的过氧化氢加至反式Pt(PEt 3)2 Cl(R)[ 1(R = 9-菲基),2(R = 4-三氟甲基苯基)]可以生成反式Pt(PEt 3)2( Cl)(OOH)(OH)(R)[ 5(R = 9-菲基),4(R = 4-三氟甲基苯基)],其中氢过氧配体反式为R。络合物5不稳定并与溶剂CH 2反应CL 2,得到的反式,顺式-Pt(PET 3)2(Cl)的2(OH)(9-菲基)(3)。用HCl处理4可获得类似的反式-顺式-Pt(PEt 3)2(Cl)2(OH)(4-三氟甲基苯基)(6)和HBr产生反式-Pt(PEt 3)2(Br)(Cl)( OH)(4-三氟甲基苯基)(7),其中Br和4-三氟甲基苯基配体是反式的。3或6在313或380 nm处发生光解会导致反式Pt(PEt 3)2 Cl(R)(1或2)。未检测到预期的副产物HOCl,但显示出真实的HOCl溶液在反应条件下会分解。在光解苯溶液中检测到氯苯和其他可将PPh
-
Age Dependence of Laryngeal Chemoreflex in Puppies作者:Han-Q Park、Won-Pyo Hong、Kwang-Moon Kim、Myung-Sang Kim、Young-Ho Kim、Dong-Young KimDOI:10.1177/000348940111001012日期:2001.10
Previously collected data have indicated that the laryngeal chemoreflex (lcr) response is exaggerated during a critical period of postnatal development in several experimental animals. These animals had fewer anatomic and physiological similarities to humans than do puppies. This investigation of the lcr in 14 anesthetized puppies was undertaken to determine age-related differences in the response to stimulation of the supraglottic laryngeal mucosa by saline solution, distilled water, cow's milk, and acid at pH 1.0. The dogs were divided into 3 age groups: group 1 consisted of 4 dogs that were 2 weeks old, and in groups 2 and 3 there were 5 puppies each, of 4 and 6 weeks of age, respectively. The lcr response (laryngospasm, apnea, respiratory depression, and bradycardia) was found in the puppies only after stimulation of the laryngeal mucosa with acid at pH 1.0, and it was more easily achieved in the 4- and 6-week age groups than in the 2-week group. These findings suggest that the lcr is an age-dependent response that appears in dogs only after 2 weeks of age. The important implication of this finding is that postnatal neural maturation may influence the laryngeal reflex in humans to some extent.
先前收集的数据表明,在几种实验动物的出生后发育的关键时期,喉化学反射(lcr)反应被夸大。这些动物与幼犬相比,与人类的解剖和生理相似性较少。对14只麻醉幼犬进行了喉化学反射的研究,以确定对盐水、蒸馏水、牛奶和pH 1.0的酸刺激喉上部喉粘膜的反应存在年龄相关差异。狗被分为3个年龄组:第1组包括4只2周大的狗,第2组和第3组分别有5只4周大和6周大的幼犬。只有在用pH 1.0的酸刺激喉粘膜后,幼犬才出现喉化学反射(喉痉挛、呼吸暂停、呼吸抑制和心动过缓),而在4周和6周龄组比2周龄组更容易实现这种反应。这些发现表明,喉化学反射是一种年龄相关的反应,只有在幼犬满2周大后才出现。这一发现的重要含义是,出生后神经成熟可能在一定程度上影响人类的喉反射。
表征谱图
-
氢谱1HNMR
-
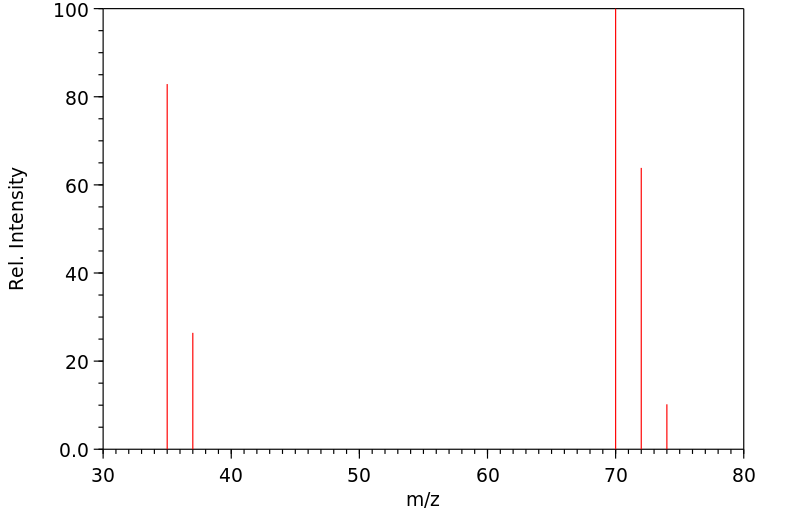
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息