毒理性
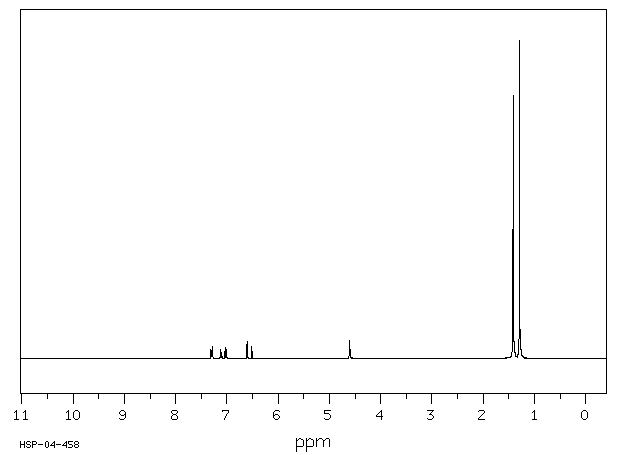
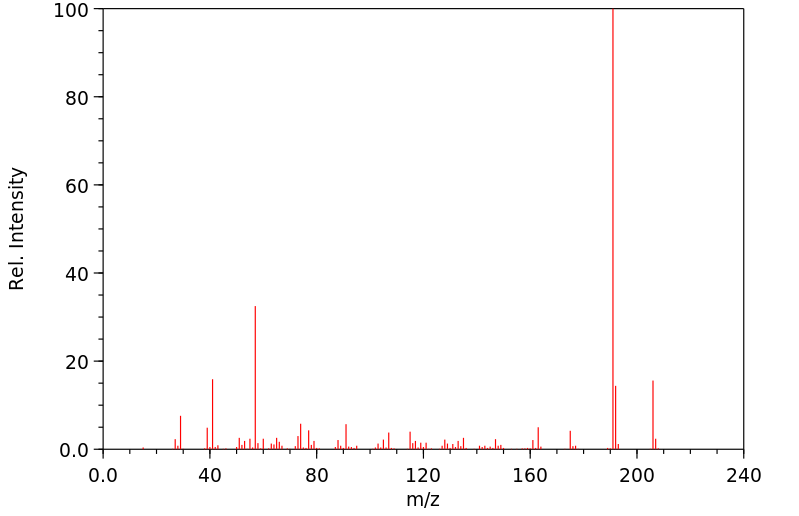
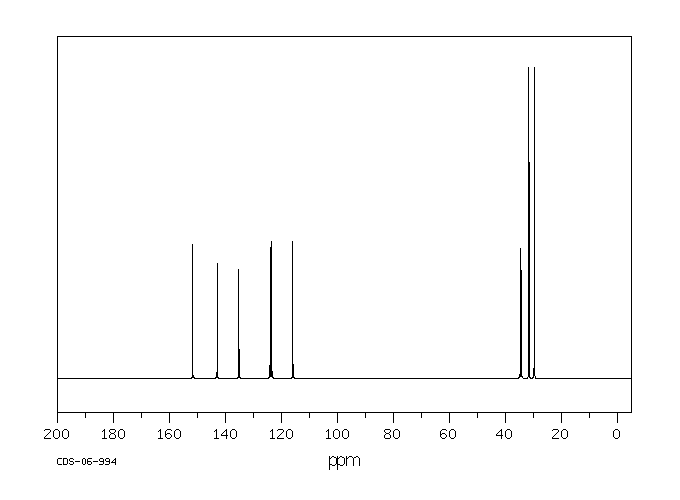
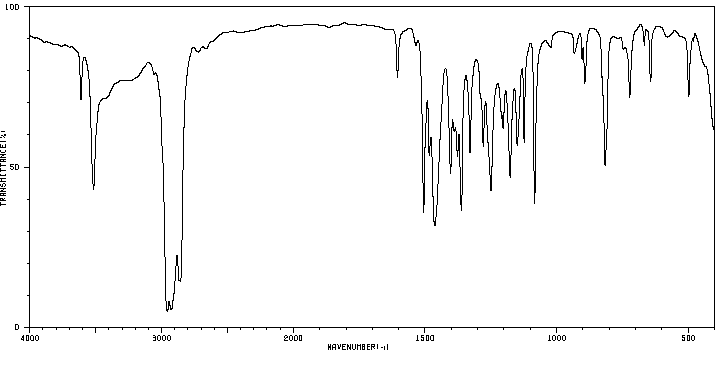
识别与用途:2,4-二叔丁基苯酚(DTBP)是一种固体,用作基于烃类产品的紫外线稳定剂和抗氧化剂。人类研究:处理含有DTBP的橡胶的工人中报告了职业性白癜风(皮肤脱色素)病例。报告了DTBP对多种人类细胞系的细胞毒性。DTBP通过上调p21和Rb以及增加β-半乳糖苷酶活性,诱导人胃腺癌AGS细胞衰老。DTBP还诱导有丝分裂灾难并产生多核细胞,这伴随着聚合微管比例的增加,可能是由于HDAC6酶活性受到抑制。动物研究:将测试材料半封闭地应用于三只兔子的完整皮肤4小时,产生了明确的红斑和轻微至轻度水肿。其他观察到的皮肤反应包括真皮毛细血管出血、苍白、表皮浅棕色变色、结痂形成和硬化浅棕色痂皮。对豚鼠无致敏性。新生大鼠对DTBP的敏感性比幼年大鼠高4-5倍。在大鼠中进行了第一代生育力/重复剂量毒性研究。亲代大鼠在交配前4周以及整个交配、妊娠和哺乳期间,分别喂食含有DTBP剂量水平为50、150或300 mg/kg/天的饮食。每组20只F1代的后代在断奶后13周内继续食用实验饮食。亲代动物的生育能力在摄入DTBP剂量水平高达300 mg/kg/天时未受影响。摄入300 mg DTBP/kg/天显著减少了出生后代的平均数量,并降低了F1代在哺乳期间的成长速度。在50和150 mg/kg/天的剂量水平上,前一种效果并不明显。在150 mg/kg/天处理的后代中也观察到了生长速度的降低,这与较高的平均窝数有关。在饲养阶段,实验组中没有与物质相关的死亡。以300 mg/kg/天的剂量水平连续13周给大鼠喂食DTBP,主要对生长速度产生了毒性影响,而在150 mg/kg/天的剂量水平上,由于食物适口性降低而引起了生长迟缓。DTBP在体内不具有断裂作用。
IDENTIFICATION AND USE: 2,4-Di-tert-butylphenol (DTBP) is a solid, which is used as UV stabilizer and an antioxidant for hydrocarbon-based products. HUMAN STUDIES: Occupational vitiligo (cutaneous depigmentation) cases have been reported in workers who handled the rubber containing DTBP. Cytoxicity to multiple human cell lines have been reported. DTBP induces senescence in human gastric adenocarcinoma AGS cells as evidenced by upregulation of p21 and Rb and increased beta-galactosidase activity. DTBP also induces mitotic catastrophe and generates multinucleated cells, which is accompanied by an increase in the proportion of polymerized tubulin, possibly caused by inhibition of HDAC6 enzyme activity. ANIMAL STUDIES: A single 4 -hour, semi-occluded application of the test material to the intact skin of three rabbits produced well-defined erythema and very slight to slight edema. Other skin reactions noted were hemorrhage of the dermal capillaries, blanching, light brown discoloration of the epidermis, crust formation and hardened light brown-colored scabs. It was non-sensitizing to guinea pigs. The susceptibility of newborn rats to DTBP was found to be 4-5 times higher than that of young rats. The 1 -generation fertility / Repeated dose toxicity study was carried out in the rat. The parental generation rats were fed diets containing DTBP at dose levels of 50, 150 or 300 mg/kg/day for 4 weeks before mating and throughout mating, gestation and lactation. Groups of 20 generation F1 progeny of each sex were maintained on the experimental diets for 13 weeks after weaning. Reproductive capability of the parental generation animals was unimpaired by the ingestion of DTBP at dose levels of up to 300 mg/kg/d. Ingestion of 300 mg DTBP/kg/d elicited a significant reduction in the mean number of progeny born and reduced the growth rate of the F1 progeny during lactation. The former effect was not apparent at dose levels of 50 and 150 mg/kg/d. The reduced growth rate was also apparent in the progeny of animals treated at 150 mg/kg/d and was associated with a higher average litter number. There were no substance related deaths in any of the experimental groups during the feeding phase. Dietary administration of DTBP at a dose level of 300 mg/kg daily for 13 weeks to rats elicited a primary toxic effect on growth rate, and at dose level of 150 mg/kg/d, elicited growth retardation which was secondary to reduced diet palatability. DTBP was not clastogenic in vivo.
来源:Hazardous Substances Data Bank (HSDB)