2,4-二叔丁基苯硫酚 | 19728-43-9
中文名称
2,4-二叔丁基苯硫酚
中文别名
2,4-二叔丁基噻吩;2,3-二甲基己烷
英文名称
2,4-di-tert-butylthiophenol
英文别名
2,4-ditert-butylbenzenethiol
CAS
19728-43-9
化学式
C14H22S
mdl
MFCD00041446
分子量
222.395
InChiKey
ACJAQOIIEMFTGS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:274.1±29.0 °C(Predicted)
-
密度:0.944±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.4
-
重原子数:15
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.571
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S23,S24/25
-
海关编码:2930909090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-{[2-(bromomethyl)-4,6-di-tert-butylphenyl]sulfanyl}propanenitrile 1451064-90-6 C18H26BrNS 368.381
反应信息
-
作为反应物:描述:2,4-二叔丁基苯硫酚 在 正丁基锂 、 四甲基乙二胺 、 potassium carbonate 作用下, 以 四氢呋喃 、 乙醚 、 正己烷 为溶剂, 反应 43.0h, 生成 3-{[2,4-di-tert-butyl-6-(hydroxymethyl)phenyl]sulfanyl}propanenitrile参考文献:名称:基于 atrans-cyclooctane-1,2-diyl(thio) 平台的 [SSSS] 型双(苯硫酚)配体的合成以及与铂配合物意外反应生成硫化物-双(硫醇基)PtII 络合物摘要:通过反式-环辛烷-1,2-二硫醇与2 mol当量的反应,制备了一种基于反式-环辛烷-1,2-二基(硫代)平台的新型[SSSS]型双(苯硫酚)配体。3,5-二叔丁基-2-(2-氰基乙硫基)苄基溴,然后去保护。[SSSS] 型配体与 [Pt(nb)(PPh3)2] (nb=降冰片烯) 或 [PtCl2(PPh3)2] 复合物的反应出人意料地产生了 [硫化物-双(硫醇基)]-PPh3 PtII 复合物伴随着 2-硫烷基苄基之一的丢失。PtII 复合物的结构由 X 射线晶体学确定。讨论了 PtII 复合物的物理性质和形成机制。图形概要DOI:10.1080/17415993.2013.781605
-
作为产物:描述:1-bromo-2,4-di-tert-butylbenzene 在 magnesium 、 sulfur 作用下, 以 四氢呋喃 为溶剂, 反应 10.75h, 以94%的产率得到2,4-二叔丁基苯硫酚参考文献:名称:一电子氧化的Ni(II)-(二硫代水杨基二胺)配合物的分子和电子结构:Ni(III)-硫醇盐对Ni(II)-噻吩基自由基状态。摘要:二硫代水杨基二胺Ni II配合物[Ni(L)](R = tBu,R'= CH2C(CH3)2CH2 1,1,R'= C6H4 2; R = H,R'= CH2C(CH3)2CH2 3,R'= C6H4 4)已通过四面体配合物[Zn(L)]的金属转移制备(R = tBu,R'= CH2C(CH3)2CH2 7,R'= C6H4 8; R = H,R'= CH2C(CH3)2CH2在图9中,R′= C 6 H 4 10)是在Zn II盐存在下,通过2,4-二-R-硫代水杨醛与二胺H 2 N-R′-NH 2缩合形成的。反磁性单核络合物[Ni(L)]显示扭曲的N2S2平面,并具有1H和13C NMR和UV / Vis光谱学特征以及通过单晶X射线晶体学表征。循环伏安法和库仑测量法已经确定,在硫酚酸酯配体上结合了tBu功能的配合物1和2经历了可逆的单电子氧化过程,而复合物3和4的类似氧化还原过程是不可DOI:10.1002/chem.200701108
文献信息
-
制备具降血糖作用的三硫代碳酸甲酯苯酯类化合物的方法
-
Evaluation of P<sub>1</sub>'-Diversified Phosphinic Peptides Leads to the Development of Highly Selective Inhibitors of MMP-11作者:Magdalini Matziari、Fabrice Beau、Philippe Cuniasse、Vincent Dive、Athanasios YiotakisDOI:10.1021/jm0308491日期:2004.1.1Phosphinic peptides were previously reported to be potent inhibitors of several matrixins (MMPs). To identify more selective inhibitors of MMP-11, a matrixin overexpressed in breast cancer, a series of phosphinic pseudopeptides bearing a variety of P-1'-side chains has been synthesized, by parallel diversification of a phosphinic template. The potencies of these compounds were evaluated against a set of seven MMPs (MMP-2, MMP-7, MMP-8, MMP-9, MMP-11, MMP-13, and MMP-14). The chemical strategy applied led to the identification of several phosphinic inhibitors displaying high selectivity toward MMP-11. One of the most selective inhibitors of MMP-11 in this series, compound 22, exhibits a K-i value of 0.23 PM toward MMP-11, while its potency toward the other MMPs tested is 2 orders of magnitude lower. This remarkable selectivity may rely on interactions of the P-1'-side chain atoms of these inhibitors with residues located at the entrance of the S-1'-cavity of MMP-11. The design of inhibitors able to interact with residues located at the entrance of MMPs' S-1'-cavity might represent an alternative strategy to identify selective inhibitors that will fully differentiate one MMP among the others.
-
Rundel,W., Chemische Berichte, 1968, vol. 101, p. 2956 - 2962作者:Rundel,W.DOI:——日期:——
-
Preparation of New<i>tert</i>-Butyl Substituted Coumarins, Thiocoumarins and Dithiocoumarins作者:Jürgen Voss、Ronald Edler、Gunadi AdiwidjajaDOI:10.1080/10426500701340949日期:2007.6.146-tert-Butyl-4-methyl- and 6,8-di-tert-butyl-4-methylcoumarin were prepared from tert-butylphenols and diketene via the corresponding aryl acetoacetates. 6-tertButyl-4-methyl-thiocoumarin (6) was obtained from 6-tert-butylthiophenol. Thionation with LAWESSON's or DAVY's reagent led to the related thion- and dithiocoumarins. The structures were proved by NMR spectroscopy and an X-ray structure analysis of 6.
-
Pohl, S.; Opitz, U.; Haase, D., Zeitschrift fur Anorganische und Allgemeine Chemie作者:Pohl, S.、Opitz, U.、Haase, D.、Saak, W.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
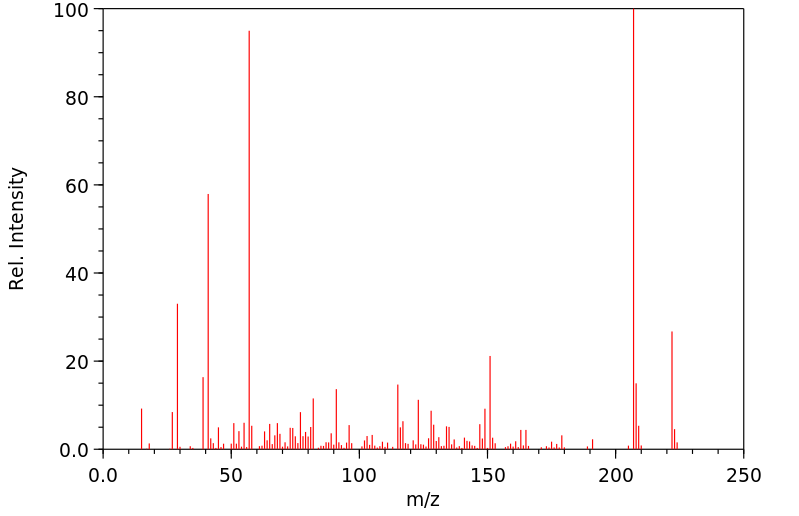
质谱MS
-
碳谱13CNMR
-
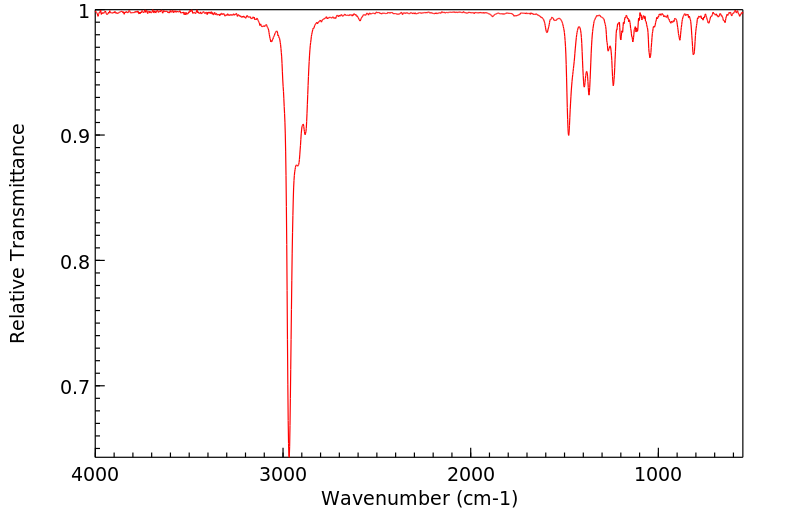
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫