德国春黄菊油 | 520-36-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>300 °C (lit.)
-
沸点:333.35°C (rough estimate)
-
密度:1.2319 (rough estimate)
-
溶解度:二甲基亚砜:27 毫克/毫升
-
LogP:3.020
-
物理描述:Solid
-
颜色/状态:Yellow needles from aqueous pyridine
-
蒸汽压力:1.01X10-10 mm Hg at 25 °C (est)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
解离常数:pKa1 = 7.12 (phenol); pKa2 = 8.10 (phenol) (est)
-
碰撞截面:156 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:20
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:87
-
氢给体数:3
-
氢受体数:5
ADMET
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2942000000
-
危险品运输编号:OTH
-
RTECS号:LK9276000
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
SDS
制备方法与用途
芹菜素是一种生物类黄酮化合物,可以在多种植物和草药中找到。在甘菊茶中含量特别丰富,并且在高剂量服用时能够起到减轻焦虑的作用。在更高剂量下,它还具有镇静效果。
理化性质芹菜素为黄色针状结晶(吡啶水溶液),熔点约为345℃~350℃。几乎不溶于水,易溶于热乙醇,并可溶解于稀氢氧化钾(KOH)溶液中。它具有利尿、调节血压、抗菌和消炎等作用。
植物来源芹菜素是一种天然存在的黄酮类化合物,主要存在于多种植物中,尤其是伞形科植物旱芹。其他如洋甘菊、蜜蜂花、紫苏、马鞭草及西洋蓍草也含有该成分。洋甘菊原产于英国,在德国、法国和摩洛哥等地也有种植。罗马洋甘菊与德国洋甘菊具有相似特征:约30公分高,中心黄色,花瓣白色,叶片略带毛绒感。洋甘菊有助于改善睡眠质量,缓解炎症及疼痛症状,并能减轻神经性皮肤瘙痒引起的失眠。中国也广泛栽培用于药用和制茶,日常凉茶的成分中常含有芹菜素,主要来自母菊洋甘菊干花。
信息由Chemicalbook的晓楠编辑整理。
生物活性与药理作用芹菜素独特的分子结构赋予了其多种生理效应及生物学特性。它是天然抗氧化剂,具有降血压、舒张血管、预防动脉粥样硬化和抑制肿瘤等作用。与其他黄酮类物质相比(如槲皮素和山奈黄酮),芹菜素具有低毒性和无诱变性等特点,在医药和食品行业中都有一定应用。
然而,由于其水溶性较差且肠道吸收率较低,导致口服生物利用度和体内活性都相对有限,限制了其在某些方面的广泛应用。
化学性质芹菜素几乎不溶于水,但能部分溶解于热乙醇,并可溶于稀氢氧化钾(KOH)溶液。它源自伞形科植物旱芹(Apium graveolens L. var. dulce DC)。
用途作为一种黄酮类化合物,芹菜素能够捕获细胞周期的G2/M期并抑制细胞扩增。通过阻断细胞周期和诱导凋亡来抑制生长似乎与p53基因的表达有关。此外,它还能抑制蛋白激酶C,并通过这一途径抑制由PMA介导的肿瘤促进作用。研究表明芹菜素还能够抑制拓扑异构酶Ⅰ催化的脱氧核糖核酸再连接并增强间隙连接细胞间的通讯。
分类有毒物质
刺激数据轻微过敏性
可燃性危险特性热分解,产生辛辣刺激烟雾
储运特性库房需低温通风干燥保存
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 白杨素 5,7-dihydroxy-2-phenyl-chromen-4-one 480-40-0 C15H10O4 254.242 金合欢素 5,7-dihydroxy-2-(4'-methoxyphenyl)-4H-1-benzopyran-4-one 480-44-4 C16H12O5 284.268 5-羟基-4',7-二甲氧基黄酮 5-hydroxy-7,4'-dimethoxyflavone 5128-44-9 C17H14O5 298.295 5-羟基黄酮 5-Hydroxyflavon 491-78-1 C15H10O3 238.243 5,7,4-三甲氧基黄酮 4',5,7-trimethoxyflavone 5631-70-9 C18H16O5 312.322 —— 7-O-lactoyl-apigenin 126462-89-3 C18H14O7 342.305 3,4,5,7-四甲氧基黄酮 2-(3,4-dimethoxyphenyl)-5,7-dimethoxy-4H-chromen-4-one 855-97-0 C19H18O6 342.348 5,7-二-(苄氧基)-2-(4-(苄氧基)苯基)-4H-苯并吡喃-4-酮 5,7-bis(benzyloxy)-2-(4-(benzyloxy)phenyl)-4H-chromen-4-one 96333-59-4 C36H28O5 540.615 黄酮 FLAVONE 525-82-6 C15H10O2 222.243 —— 6-bromo-5-hydroxy-7,4'-dimethoxyflavone 35095-48-8 C17H13BrO5 377.191 —— chrysin 4'-O-α-D-6-deoxyallopyranoside 1033607-50-9 C21H20O9 416.384 —— apigenin 4'-O-α-D-glucopyranoside 1256165-03-3 C21H20O10 432.384 —— apigenin-4'-O-β-D-glucopyranoside 20486-34-4 C21H20O10 432.384 5-羟基-2-(4-羟基苯基)-4-氧代-4H-苯并吡喃-7-基alpha-D-吡喃葡萄糖苷 apigenin-7-O-glucoside 94159-34-9 C21H20O10 432.384 芹甙元-7-葡萄糖苷 apigenin 7-O-glucoside 578-74-5 C21H20O10 432.384 —— apigenin 7-O-D-glucoside 495417-72-6 C21H20O10 432.384 —— apigenin-7-O-β-D-allopyranoside 527704-27-4 C21H20O10 432.384 —— apigenin 7-O-galactoside 23598-21-2 C21H20O10 432.384 —— apigenin 7-O-<2-O-β-glucopyranosyl-lactate> —— C24H24O12 504.447 —— 7-O-α-L-rhamnopyranosyl-4'-O-rutinosylapigenin 120282-90-8 C33H40O18 724.67 5-O-beta-D-吡喃葡萄糖苷芹菜甙元 apigenin 5-O-β-D-glucopyranoside 28757-27-9 C21H20O10 432.384 —— apigenin 4'-O-β-D-glucuronopyranoside —— C21H18O11 446.367 野漆树苷 rhoifolin 17306-46-6 C27H30O14 578.527 —— apigenin-7-O-neohesperidoside —— C27H30O14 578.527 —— 7-(O-6-deoxy-α-L-mannpyranosyl-(1→2)-[O-6-deoxy-α-L-mannopyranosyl-(1→6)]-β-D-glucopyranosyl)oxo-5-hydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one 260413-62-5 C33H40O18 724.67 —— crotonoylcosmosiin 123656-62-2 C25H24O11 500.459 —— apigenin-4'-(6-O-(p-(Z)-coumaroyl)-β-D-glucopyranoside) 1198404-32-8 C30H26O12 578.529 —— apigenin 7-(4-O-β-glucosyl-trans-p-coumarate) 126871-97-4 C30H26O12 578.529 芹菜素-7-葡萄糖醛酸 apigenin-7-O-β-D-glucuronide 29741-09-1 C21H18O11 446.367 —— apigenin-7-glucuronic acid 29741-09-1 C21H18O11 446.367 —— apigenin 7-O-β-D-galacturonopyranoside 56317-11-4 C21H18O11 446.367 —— Apigenin-7-o-glucuronide —— C21H18O11 446.4 —— apigenin 5-O-α-l-rhamnopyranosyl-(1-> 3)-β-D-glucopyranoside —— C27H30O14 578.527 —— apigenin-4'-(3-O-(p-(E)-coumaroyl)-β-D-glucopyranoside) 1198404-33-9 C30H26O12 578.529 芹菜甙 apiin 26544-34-3 C26H28O14 564.5 —— apigenin-7-galacturonic acid methyl ester —— C22H20O11 460.394 —— apigenin-4'-(3,6-di-O-(p-(E)-coumaroyl)-β-D-glucopyranoside) 1198404-34-0 C39H32O14 724.675 —— apigenin 5-O-α-L-rhamnopyranosyl-(1'''->2'')-6''-O-acetyl-β-D-glucopyranoside 125300-52-9 C29H32O15 620.564 —— apigenin 7-O-[3''-O-trans-p-coumaroyl]-β-D-glucopyranoside —— C30H26O12 578.529 海常黄甙 apigenin 7-O-[β-D-glucuronopyranosyl-(1->2)]-O-β-D-glucuronopyranoside 119738-57-7 C27H26O17 622.493 —— 7-[(6-O-(2E-3-(4-hydroxyphenyl)-1-oxo-2-propenyl)-β-D-glucopyranosyl)]-(1->2)-[(6-deoxy-α-L-mannopyranosyl)]-(1->3)-[(6-deoxy-α-L-mannopyranosyl)-oxy]-5-hydroxy-2-(4'-hydroxyphenyl)-4H-1-benzopyran —— C42H46O20 870.815 —— genistin 529-59-9 C21H20O10 432.384 —— embigenin 21089-34-9 C23H24O10 460.438 —— isovitexin-6''-O-α-L-glucopyranoside —— C27H30O15 594.526 —— nivyaside 84563-91-7 C27H30O14 578.527 - 1
- 2
- 3
- 4
- 5
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 金合欢素 5,7-dihydroxy-2-(4'-methoxyphenyl)-4H-1-benzopyran-4-one 480-44-4 C16H12O5 284.268 4',5-二羟基-7-甲氧基黄酮 genkwanin 437-64-9 C16H12O5 284.268 5-羟基-4',7-二甲氧基黄酮 5-hydroxy-7,4'-dimethoxyflavone 5128-44-9 C17H14O5 298.295 木犀草素 2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on 491-70-3 C15H10O6 286.241 —— 2-[4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy]acetic acid —— C17H12O7 328.278 野黄芩素 scutellarein 529-53-3 C15H10O6 286.241 —— 7-O-allyl-apigenin 1262000-19-0 C18H14O5 310.306 —— 5,7-Dideuteriooxy-2-(4-deuteriooxyphenyl)chromen-4-one 263711-81-5 C15H10O5 273.218 —— 5-hydroxy-2-(4-hydroxyphenyl)-7-(methoxymethoxy)chromen-4-one —— C17H14O6 314.295 —— 7-(tert-butoxy)-5-hydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one 1610737-63-7 C19H18O5 326.349 —— 5-hydroxy-7-isopropoxy-2-(4-isopropoxyphenyl)-4H-chromen-4-one —— C21H22O5 354.403 5,7,4-三甲氧基黄酮 4',5,7-trimethoxyflavone 5631-70-9 C18H16O5 312.322 —— 7-Difluoromethoxy-2-(4-difluoromethoxy-phenyl)-5-hydroxy-chromen-4-one 801218-76-8 C17H10F4O5 370.257 —— 5-hydroxy-7-(methoxymethoxy)-2-(4-(methoxymethoxy)phenyl)-4H-chromen-4-one 134955-46-7 C19H18O7 358.348 —— 5,4'-dihydroxy-7-(1-carboxymethoxy)flavone —— C17H12O7 328.278 —— 5,4'-dihydroxy-7-(3-carboxypropoxy)flavone —— C19H16O7 356.332 —— 7,4'-diacetoxy-5-hydroxyflavone 857-79-4 C19H14O7 354.316 6-羟基四羟黄酮 3',4',5,6,7-pentahydroxyflavone 18003-33-3 C15H10O7 302.24 —— 7,4'-di-O-benzyl apigenin 32375-14-7 C29H22O5 450.491 —— apigenin 7-sulfate 56857-56-8 C15H10O8S 350.306 —— 5-hydroxy-4-oxo-2-[4-(propanoyloxy)phenyl]-4H-chromen-7-yl propanoate —— C21H18O7 382.37 —— 5,7-diisopropoxy-2-(4-isopropoxyphenyl)-4H-benzopyran-4-one —— C24H28O5 396.483 —— apigenin 7,4'-disulphate —— C15H10O11S2 430.37 —— Apigenin-TMS-ether 18874-97-0 C24H34O5Si3 486.787 —— 7,4'-dihydroxy-5-(1-carboxymethoxy)flavone —— C17H12O7 328.278 —— N-hydroxy-7-((5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)heptanamide —— C22H23NO7 413.427 —— 7-((4-fluorobenzyl)oxy)-5-hydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one 1610737-62-6 C22H15FO5 378.357 —— 7,4'-dihydroxy-5-(1-ethoxycarbonylmethoxy)flavone —— C19H16O7 356.332 —— 5,7-diacetoxy-4'-hydroxyflavone 132351-62-3 C19H14O7 354.316 —— 6-((2-(4-(2-(dimethylamino)ethoxy)phenyl)-5-hydroxy-4-oxo-4H-chromen-7-yl)oxy)-N-hydroxyhexanamide —— C25H30N2O7 470.522 —— 4-(5-acetoxy-7-hydroxy-4-oxo-4H-chromen-2-yl)phenylacetate 34331-04-9 C19H14O7 354.316 —— acacetin diacetate 5892-39-7 C20H16O7 368.343 [7-乙酰氧基-2-(4-乙酰氧基苯基)-4-氧代苯并吡喃-5-基]乙酸酯 apigenin triacetate 3316-46-9 C21H16O8 396.353 5,7-二-(苄氧基)-2-(4-(苄氧基)苯基)-4H-苯并吡喃-4-酮 5,7-bis(benzyloxy)-2-(4-(benzyloxy)phenyl)-4H-chromen-4-one 96333-59-4 C36H28O5 540.615 —— 5,7,8-trihydroxy-2-(4-hydroxyphenyl)-4H-benzofuro[3,2-g]chromen-4-one —— C21H12O7 376.322 8-去甲基桉树素 8-demethyleucalyptin 5689-38-3 C18H16O5 312.322 —— 7,4'-bis-O-methoxymethyl-5-O-prenylapigenin 852247-42-8 C24H26O7 426.466 —— 7,4'-diacetoxy-5-(3-methylbut-2-enyloxy)flavone 583037-97-2 C24H22O7 422.434 槲皮素 quercetol 117-39-5 C15H10O7 302.24 6-异戊烯基芹菜甙元 6-prenylapigenin 68097-13-2 C20H18O5 338.36 —— 5,9,10-trihydroxy-2-(4-hydroxyphenyl)-4H-benzofuro[2,3-h]chromen-4-one —— C21H12O7 376.322 —— 5,7,4'-tripivaloyloxyflavone —— C30H34O8 522.595 —— 5,4'-di-O-hexanoyl-apigenin 1145669-47-1 C27H30O7 466.531 —— 5,7,4'-tri-O-hexanoyl-apigenin 1145669-46-0 C33H40O8 564.676 —— 3'-nitroapigenin 890661-83-3 C15H9NO7 315.239 —— 6-(1,1-dimethylallyl)apigenin —— C20H18O5 338.36 —— 6-geranylapigenin 134955-26-3 C25H26O5 406.478 —— 8-(3,4-dihydroxyphenyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one —— C21H14O7 378.338 —— 5-hydroxy-2-(4-hydroxyphenyl)-7-morpholino-4H-chromen-4-one 1610737-74-0 C19H17NO5 339.348 —— 5,7-dihydroxy-2-(4-hydroxyphenyl-3,5-D2)-4H-1-benzopyran-4-one-3-D —— C15H10O5 273.218 去甲氧基苏打基亭 demethoxysudachitin 4323-80-2 C17H14O7 330.294 5,7-二羟基-6,8,4'-三甲氧基黄酮 nevadensin 10176-66-6 C18H16O7 344.321 —— N-((phenoxy)((5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)glycine methyl ester —— C24H20NO9P 497.398 —— 3,7-diethoxy-2-(4-ethoxy-phenyl)-5-hydroxy-chromen-4-one 744239-42-7 C21H22O6 370.402 —— 8-(6''-umbelliferyl)-apigenin —— C24H14O8 430.37 甘草黄酮C 4',5,7-trihydroxy-8-prenylflavone 72357-31-4 C20H18O5 338.36 —— N-((phenoxy)((5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)-L-alanine methyl ester —— C25H22NO9P 511.425 —— 8-iodo-5,7-diisopropoxy-2-(4-isopropoxyphenyl)-4H-chromen-4-one —— C24H27IO5 522.38 —— apigenin-4'-O-β-D-glucopyranoside 20486-34-4 C21H20O10 432.384 —— 5-Hydroxy-8-(4-hydroxyphenyl)-2,2-dimethyl-3,4-dihydropyrano[3,2-g]chromen-6-one 76288-44-3 C20H18O5 338.36 Carpachromene; 5-羟基-8-(4-羟基苯基)-2,2-二甲基-2H,6H-苯并[1,2-b:5,4-b']二吡喃-6-酮 5-hydroxy-8-(4-hydroxyphenyl)-2,2-dimethyl-2H-pyrano[3,2-g]chromen-6one 57498-96-1 C20H16O5 336.344 —— 8-(4-methylpiperazin-1-ylmethyl)-5,7-dihydroxy-2-(4-phenol)-4H-1-benzopyran-4-one 1334524-22-9 C21H22N2O5 382.416 —— 5,7-dihydroxy-2-(4-hydroxyphenyl-3,5-D2)-4H-1-benzopyran-4-one-3,6,8-D3 263711-74-6 C15H10O5 275.202 —— 4',5,7-trihydroxy-8-(pyrrolidin-1-ylmethyl)-2-phenyl-4H-chromen-4-one 1334524-20-7 C20H19NO5 353.375 —— 7,4'-diacetoxy-5-hydroxy-6-C-(1,1-dimethylallyl)flavone —— C24H22O7 422.434 6-[6-(5,7-二羟基-4-氧代苯并吡喃-2-基)-2,3-二羟基苯基]-2-(3,4-二羟基苯基)-5,7-二羟基苯并吡喃-4-酮 6-(6-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2,3-dihydroxyphenyl)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one 116383-34-7 C30H18O12 570.466 —— 6-(6-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2,3-dihydroxyphenyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one —— C30H18O11 554.466 —— 4',5,7-trihydroxy-8-(morpholinomethyl)-2-phenyl-4H-chromen-4-one 1334524-13-8 C20H19NO6 369.374 —— kaempferol 7-O-α-L-rhamnoside 88109-95-9 C21H20O9 416.384 —— N-((phenoxy)((5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)-L-valine methyl ester —— C27H26NO9P 539.478 —— N-((phenoxy)((5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)-L-leucine methyl ester —— C28H28NO9P 553.505 芹甙元-7-葡萄糖苷 apigenin 7-O-glucoside 578-74-5 C21H20O10 432.384 —— 7-(2,6-dinitro-4-(trifluoromethyl)phenoxy)-5-hydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one —— C22H11F3N2O9 504.333 —— 5,7-dihydroxy-8-((4-(hydroxymethyl)piperidin-1-yl)methyl)-2-(4-hydroxyphenyl)-4hchromen-4-one 1334524-19-4 C22H23NO6 397.428 —— 6,8-diprenylapigenin 70872-32-1 C25H26O5 406.478 —— apigenin 7-O-β-D-(4''-O-methyl)glucopyranoside —— C22H22O10 446.411 —— N-((phenoxy)((5-acetoxy-2-(4-acetoxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)glycine methyl ester —— C28H24NO11P 581.472 —— 5,7-dihydroxy-2-(4-hydroxyphenyl)-8-((3-hydroxypiperidin-1-yl)methyl)-4H-chromen-4-one 1334524-18-3 C21H21NO6 383.401 —— 7,4'-bis-O-methoxymethyl-8-prenylapigenin 852247-43-9 C24H26O7 426.466 —— 5,7-dihydroxy-2-(4-hydroxyphenyl)-8-((4-(thiophen-2-ylmethyl)piperazin-1-yl)methyl)-4H-chromen-4-one —— C25H24N2O5S 464.542 —— N-((phenoxy)((5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)-L-phenylalanine methyl ester —— C31H26NO9P 587.522 —— 4'-acetoxy-6,7-(2,2-dimethylpyrano)-5-prenyloxyflavone —— C27H26O6 446.5 —— N-((phenoxy)((5-acetoxy-2-(4-acetoxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)-L-alanine methyl ester —— C29H26NO11P 595.499 Atalantoflavone; 5-羟基-2-(4-羟基苯基)-8,8-二甲基-4H,8H-苯并[1,2-b:3,4-b']二吡喃-4-酮 atalantoflavone 119309-02-3 C20H16O5 336.344 6-[3-(2-羧基乙酰氧基)-7-(beta-D-吡喃葡萄糖基氧基)-5-羟基-4-氧代-4H-苯并吡喃-2-基]-3-羟基-2,4-环己二烯-1-基 apigenin 7-O-β-D-(6''-O-malonyl)glucopyranoside 86546-87-4 C24H22O13 518.431 —— 6′′,6′′-dimethyl-4′′,5′′-dihydropyrano[2′′,3′′:7,6]apigenin 76288-47-6 C20H18O5 338.36 —— apigenin-7-glucuronic acid 29741-09-1 C21H18O11 446.367 芹菜素-7-葡萄糖醛酸 apigenin-7-O-β-D-glucuronide 29741-09-1 C21H18O11 446.367 —— 7-(2,4-dinitro-6-(trifluoromethyl)phenoxy)-2-(4-(2,4-dinitro-6-(trifluoromethyl)phenoxy)phenyl)-5-hydroxy-4H-chromen-4-one —— C29H12F6N4O13 738.424 —— N-((phenoxy)((5-acetoxy-2-(4-acetoxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)-L-valine methyl ester —— C31H30NO11P 623.553 —— N-((phenoxy)((5-acetoxy-2-(4-acetoxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)-L-leucine methyl ester —— C32H32NO11P 637.58 芹菜素-7-O-葡萄糖醛酸甲酯苷 apigenin 7-O-β-D-glucopyranosiduronic acid methyl ester 29781-25-7 C22H20O11 460.394 —— N-((phenoxy)((5-acetoxy-2-(4-acetoxyphenyl)-4-oxo-4H-chromene-7-yl)oxy)phosphoryl)-L-phenylalanine methyl ester —— C35H30NO11P 671.597 —— 7-(3-chloro-2,6-dinitro-4-(trifluoromethyl)phenoxy)-2-(4-(3-chloro-2,6-dinitro-4-(trifluoromethyl)phenoxy)phenyl)-5-hydroxy-4H-chromen-4-one —— C29H10Cl2F6N4O13 807.314 —— isolaxifolin 144049-82-1 C25H24O5 404.463 —— 4'-acetoxy-5-hydroxy-6,7-(2,2-dimethylpyrano)-8-prenylflavone —— C27H26O6 446.5 —— 5,4'-di-O-hexanoylapigenin-7-O-2,3,4,6-tetra-O-benzoyl-β-D-glucopyranoside 1145669-48-2 C61H56O16 1045.11 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:德国春黄菊油 在 Agrocybe aegerita peroxygenase 、 双氧水 、 维生素 C 作用下, 以 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 8.0h, 以42%的产率得到野黄芩素参考文献:名称:芳香族过氧化酶对不同类黄酮的区域选择性羟化作用摘要:芳香过氧化物酶是选择性氧化多种有机化合物的细胞外真菌生物催化剂。我们发现,真菌的过氧柱状田头菇(AAE APO)催化ħ 2 ö 2依赖性多样黄酮的羟基化。反应进行迅速并区域选择性地产生优选的单羟基化产物,例如从黄烷酮,芹菜素,木犀草素,黄酮以及黄豆苷元,槲皮素,山奈酚和染料木黄酮。除了羟化,Ø充分甲氧基蜜橘素-demethylation被催化AAEAPO。该酶仅对槲皮素糖苷芦丁缺乏活性,这可能是由于庞大的糖取代基的空间位阻所致。机理研究表明,在羟基化过程中存在环氧化物中间体,并将H 2 O 2衍生的氧掺入反应产物中。我们的结果提出了真菌过氧合酶可能用于多功能,成本有效且可扩展的类黄酮代谢物合成的可能性。DOI:10.1016/j.tet.2011.05.008
-
作为产物:参考文献:名称:Flavonoid glycosides of Artemisia monosperma and A. herba-alba摘要:DOI:10.1016/s0031-9422(00)80845-6
-
作为试剂:描述:参考文献:名称:Glutathione-Dependent Generation of Reactive Oxygen Species by the Peroxidase-Catalyzed Redox Cycling of Flavonoids摘要:Catalytic concentrations of apigenin (a flavone containing a phenol B ring) and naringin or naringenin (flavanones containing a phenol B ring) caused extensive GSH oxidation at a physiological pH in the presence of peroxidase. Only catalytic H2O2 concentrations were required, indicating a redox cycling mechanism that generated H2O2 was involved. Extensive oxygen uptake ensued, the extent of which was proportional to the extent of GSH oxidation to GSSG and was markedly increased by superoxide dismutase. These results suggest that prooxidant phenoxyl radicals formed by these flavonoids co-oxidized GSH to form thiyl radicals which activated oxygen. GSH also prevented the peroxidase-catalyzed oxidative destruction of these flavonoids which suggests that phenoxyl radicals initiated the oxidative destruction. This is the first time that a group of flavonoids have been identified as prooxidants independent of autoxidation reactions catalyzed by the transition metal ions Fe3+, Fe2+, Mn2+, and CU2+.DOI:10.1021/tx980271b
文献信息
-
[EN] ALANINE-BASED MODULATORS OF PROTEOLYSIS AND ASSOCIATED METHODS OF USE<br/>[FR] MODULATEURS DE PROTÉOLYSE À BASE D'ALANINE ET PROCÉDÉS D'UTILISATION ASSOCIÉS申请人:ARVINAS INC公开号:WO2017011590A1公开(公告)日:2017-01-19The description relates to inhibitors of Apoptosis Proteins (TAPs) binding compounds, including Afunctional compounds comprising the same, which find utility as modulators of targeted ubiquitination, especially inhibitors of a variety of polypeptides and other proteins which are degraded and/or otherwise inhibited by bifunctional compounds according to the present invention. In particular, the description provides compounds, which contain on one end a ligand which binds to the IAP E3 ubiquitin ligase and on the other end a moiety which binds a target protein such that the target protein is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of that protein. Compounds can be synthesized that exhibit a broad range of pharmacological activities consistent with the degradation/inhibition of targeted polypeptides of nearly any type.
-
[EN] AMINE-LINKED C3-GLUTARIMIDE DEGRONIMERS FOR TARGET PROTEIN DEGRADATION<br/>[FR] DÉGRONIMÈRES DE C3-GLUTARIMIDE LIÉS À UNE AMINE POUR LA DÉGRADATION DE PROTÉINES CIBLES申请人:C4 THERAPEUTICS INC公开号:WO2017197051A1公开(公告)日:2017-11-16This invention provides amine-linked C3-glutarimide Degronimers and Degrons for therapeutic applications as described further herein, and methods of use and compositions thereof as well as methods for their preparation.这项发明提供了胺连接的C3-戊二酰亚胺Degronimers和Degrons,用于治疗应用,如本文进一步描述的,以及它们的使用方法、组合物以及它们的制备方法。
-
Curcumin analogs with anti-tumor and anti-angiogenic properties申请人:——公开号:US20020019382A1公开(公告)日:2002-02-14The present invention is directed to curcumin analogs exhibiting anti-tumor and anti-angiogenic properties, pharmaceutical formulations including such compounds and methods of using such compounds.本发明涉及表现出抗肿瘤和抗血管生成特性的姜黄素类似物,包括这些化合物的药物配方以及使用这些化合物的方法。
-
Accurate Prediction of Glucuronidation of Structurally Diverse Phenolics by Human UGT1A9 Using Combined Experimental and In Silico Approaches作者:Baojian Wu、Xiaoqiang Wang、Shuxing Zhang、Ming HuDOI:10.1007/s11095-012-0666-z日期:2012.6Catalytic selectivity of human UGT1A9, an important membrane-bound enzyme catalyzing glucuronidation of xenobiotics, was determined experimentally using 145 phenolics and analyzed by 3D-QSAR methods. Catalytic efficiency of UGT1A9 was determined by kinetic profiling. Quantitative structure activity relationships were analyzed using CoMFA and CoMSIA techniques. Molecular alignment of substrate structures was made by superimposing the glucuronidation site and its adjacent aromatic ring to achieve maximal steric overlap. For a substrate with multiple active glucuronidation sites, each site was considered a separate substrate. 3D-QSAR analyses produced statistically reliable models with good predictive power (CoMFA: q2 = 0.548, r2 = 0.949, r pred 2 = 0.775; CoMSIA: q2 = 0.579, r2 = 0.876, r pred 2 = 0.700). Contour coefficient maps were applied to elucidate structural features among substrates that are responsible for selectivity differences. Contour coefficient maps were overlaid in the catalytic pocket of a homology model of UGT1A9, enabling identification of the UGT1A9 catalytic pocket with a high degree of confidence. CoMFA/CoMSIA models can predict substrate selectivity and in vitro clearance of UGT1A9. Our findings also provide a possible molecular basis for understanding UGT1A9 functions and substrate selectivity.通过实验使用145种酚类化合物,并通过3D-QSAR方法分析,确定了人UGT1A9的催化选择性。UGT1A9是一种重要的膜结合酶,催化外源性物质的葡糖醛酸化反应。通过动力学分析确定了UGT1A9的催化效率。使用CoMFA和CoMSIA技术分析了定量结构活性关系。通过将葡糖醛酸化位点及其相邻的芳香环重叠,实现了底物结构的最大立体重叠。对于具有多个活性葡糖醛酸化位点的底物,每个位点被视为单独的底物。3D-QSAR分析产生了统计上可靠的模型,具有良好的预测能力(CoMFA:q2=0.548,r2=0.949,r pred 2=0.775;CoMSIA:q2=0.579,r2=0.876,r pred 2=0.700)。通过轮廓系数图阐明了底物中负责选择性差异的结构特征。将轮廓系数图叠加在UGT1A9的同源模型的催化口袋中,能够高度自信地识别UGT1A9的催化口袋。CoMFA/CoMSIA模型可以预测底物的选择性和UGT1A9的体外清除率。我们的发现还提供了理解UGT1A9功能和底物选择性的可能分子基础。
-
[EN] MACROMOLECULAR PRODRUG-BASED THERMOSENSITIVE INJECTABLE GEL AS A NOVEL DRUG DELIVERY PLATFORM<br/>[FR] GEL INJECTABLE THERMOSENSIBLE À BASE DE PRO-MÉDICAMENT MACROMOLÉCULAIRE EN TANT QUE NOUVELLE PLATEFORME D'ADMINISTRATION DE MÉDICAMENT申请人:UNIV NEBRASKA公开号:WO2020087057A1公开(公告)日:2020-04-30This application discloses prodrug-based thermosensitive gel ("ProGel") comprised of conjugates of dmg molecules with water-soluble polymeric carriers, which are capable of controlled release of the dmg molecules into the tissue of a subject. Use of the ProGel-Drug conjugates for treatment of various diseases or disorders and methods of preparing them are also disclosed.这种应用披露了基于前药的热敏凝胶(“ProGel”),由dmg分子与水溶性聚合物载体的共轭物组成,能够将dmg分子控制释放到受试者的组织中。还披露了将ProGel-药物共轭物用于治疗各种疾病或紊乱的方法以及制备它们的方法。
表征谱图
-
氢谱1HNMR
-
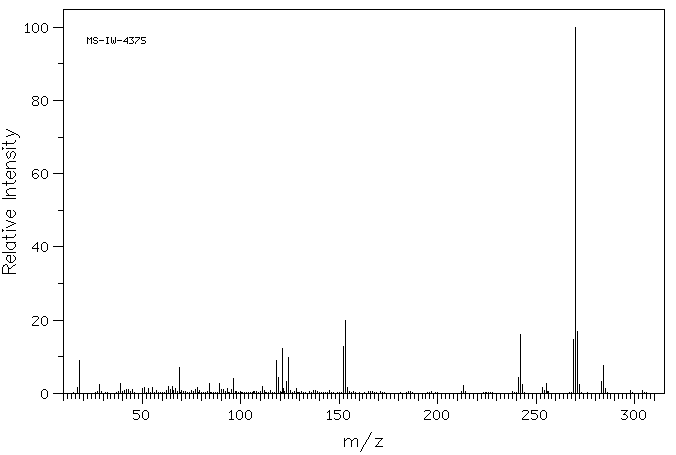
质谱MS
-
碳谱13CNMR
-
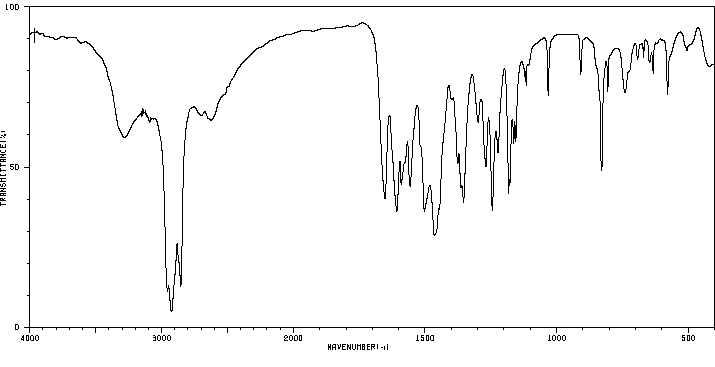
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息