代谢
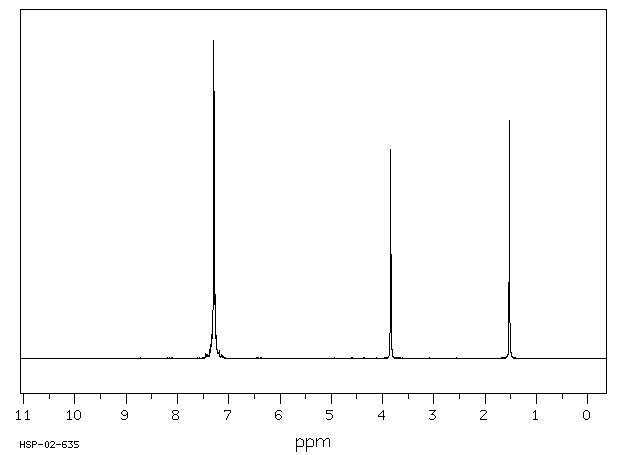
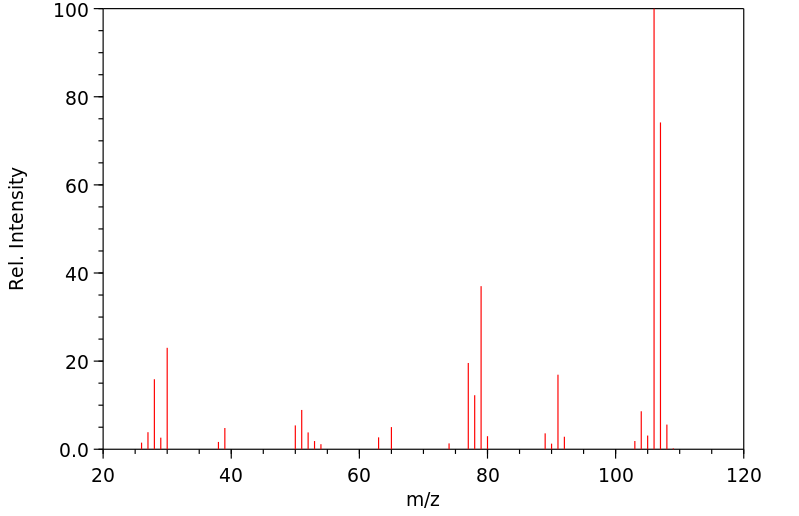
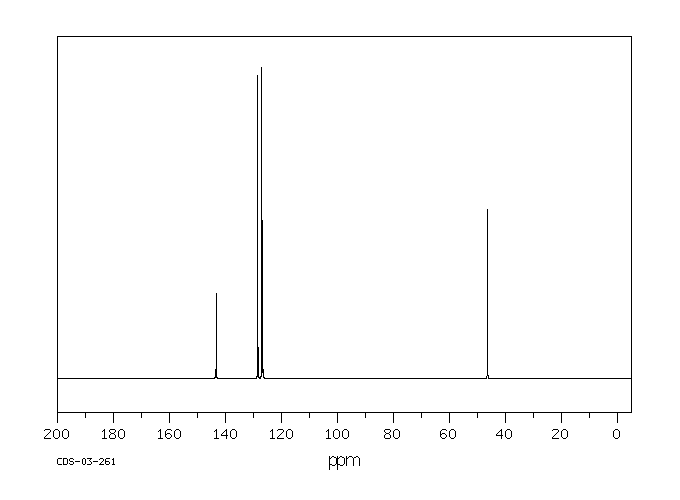
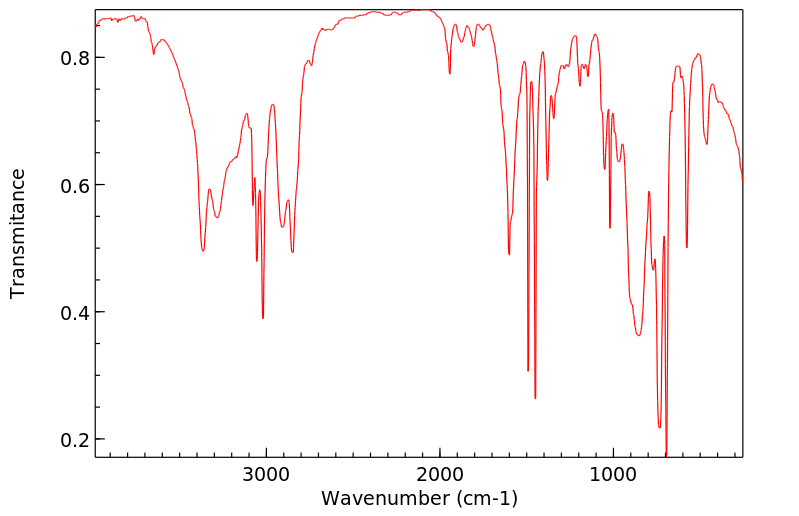
在大鼠体内和体外对苯甲基胺的处置进行了研究。苯甲基胺仅被大鼠肝脏亚细胞组分代谢到很小的程度。相比之下,它在体内大鼠中广泛代谢。使用稳定同位素标记的苯甲基胺进行的体内研究使得能够快速通过质谱识别大鼠胆汁和尿液中存在的代谢物。苯甲基胺的主要代谢物是通过苯甲酸与甘氨酸结合形成的马尿酸。对给予等摩尔混合物(d(0):d(7)-或d(0):d(2)-苯甲基胺)的大鼠的胆汁和尿液进行LC/MS分析,除了马尿酸代谢物外,还发现了几种谷胱甘肽加成物。各种谷胱甘肽加成物的存在表明苯甲基胺被代谢为多种活性中间体。发现了几种代谢途径,包括那些独立于P450的途径,产生了这些中间体。一个以前未记录的途径包括形成一个新的碳-氮键,导致可能产生反应性中间体Ar-CH(2)-NH(CO)-X,能够与各种亲核试剂发生相互作用。这种活性中间体的来源推测是通过形成甲酰胺或碳酸酸代谢物。由这种中间体Ar-CH(2)-NH(CO)-X与亲核试剂反应产生的代谢物包括S-[苄基碳酰胺]谷胱甘肽、N-乙酰-S-[苄基碳酰胺]半胱氨酸、S-[苄基碳酰胺]半胱氨酰甘氨酸、S-[苄基碳酰胺]半胱氨酰谷氨酸、N-[苄基碳酰胺]谷氨酸和一个氧化的谷胱甘肽加成物。通过这条途径激活胺类尚未见诸报道。苯甲基胺的氧化脱氨产生苯甲醛被证明是马尿酸代谢物和S-苄基-L-谷胱甘肽的前体。S-苄基-L-谷胱甘肽结合物的形成表明苯甲基胺中的胺基被移位,随后在苄基位置加入了谷胱甘肽。除了这些新途径之外,还鉴定了由于环氧化物的形成而形成的其他几种谷胱甘肽衍生的加成物。证实了苯甲基胺被大鼠P450 2A1和2E1转化为苯甲酰胺,后者迅速代谢为环氧化物。提出了形成苯甲基胺各种GSH加成物的机制。
The in vivo and in vitro disposition of benzylamine was investigated in rats. Benzylamine was metabolized to only a small extent by rat liver subcellular fractions. In contrast, it was extensively metabolized in vivo in rats. In vivo studies performed with stable isotope-labeled benzylamine enabled rapid mass spectrometric identification of metabolites present in rat bile and urine. The major metabolite of benzylamine was the hippuric acid formed by glycine conjugation of benzoic acid. LC/MS analysis of bile and urine obtained from rats dosed with 1:1 equimolar mixture of either d(0):d(7)- or d(0):d(2)-benzylamine showed the presence of several glutathione adducts in addition to the hippuric acid metabolite. The presence of various glutathione adducts indicated that benzylamine was metabolized to a number of reactive intermediates. Various metabolic pathways, including those independent of P450, were found to produce these intermediates. A previously undocumented pathway included the formation of a new carbon-nitrogen bond that led to a potentially reactive intermediate, Ar-CH(2)-NH(CO)-X, capable of interacting with various nucleophiles. The origin of this reactive intermediate is postulated to occur via the formation of either a formamide or carbamic acid metabolites. Metabolites which were produced by the reaction of this intermediate, Ar-CH(2)-NH(CO)-X with nucleophiles included S-[benzylcarbamoyl] glutathione, N-acetyl-S-[benzylcarbamoyl]cysteine, S-[benzylcarbamoyl] cysteinylglycine, S-[benzylcarbamoyl] cysteinylglutamate, N-[benzylcarbamoyl] glutamate, and an oxidized glutathione adduct. Bioactivation of amines via this pathway has not been previously described. The oxidative deamination of benzylamine yielding the benzaldehyde was demonstrated to be a precursor to the hippuric acid metabolite and S-benzyl-L-glutathione. The formation of the S-benzyl-L-glutathione conjugate showed that a net displacement of amine from benzylamine had taken place with a subsequent addition of glutathione at the benzylic position. In addition to these novel pathways, a number of other glutathione-derived adducts formed as a result of epoxide formation was characterized. It was demonstrated that benzylamine was converted by rat P450 2A1 and 2E1 to benzamide that was rapidly metabolized to an epoxide. Mechanisms are proposed for the formation of various GSH adducts of benzylamine.
来源:Hazardous Substances Data Bank (HSDB)