苯甲基苯胺 | 103-32-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:35-38 °C (lit.)
-
沸点:306-307 °C (lit.)
-
密度:1.061 g/mL at 25 °C (lit.)
-
闪点:217 °F
-
溶解度:酒精:可溶
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.076
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29214980
-
危险品运输编号:UN 2577 8/PG 2
-
储存条件:储存于阴凉、通风的库房。远离火种、热源,应与氧化剂分开存放,切忌混储。配备相应种类和数量的消防器材。储区内应备有泄漏应急处理设备及合适的收容材料。
SDS
模块 1. 化学品
1.1 产品标识符
: N-苄基苯胺
产品名称
1.2 鉴别的其他方法
N-Phenylbenzylamine
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: N-Phenylbenzylamine
别名
: C13H13N
分子式
: 183.25 g/mol
分子量
组分 浓度或浓度范围
N-Benzylaniline
<=100%
化学文摘登记号(CAS 103-32-2
No.) 203-100-4
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 凝结成块或碎片
颜色: 黄色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 35 - 38 °C - lit.
f) 沸点、初沸点和沸程
306 - 307 °C - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
1.061 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
酸, 酰基氯, 酸酐, 氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: 3335
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: Aviation regulated solid, n.o.s. (N-Benzylaniline)
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: 9
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
N-苄基苯胺是抗组胺药安他唑啉及其他N-取代的苄基苯胺的主要代谢物。
化学性质N-苄基苯胺为柱状结晶,熔点在37-38℃之间,沸点在306-307℃,也可在201-203℃(4.93kPa)下蒸馏。闪点为152℃,相对密度为1.065(25/4℃)。该物质不溶于水,但易溶于乙醇、乙醚和氯仿。
用途 生产方法生产N-苄基苯胺的过程如下:首先将碳酸氢钠、水与苯胺混合并搅拌,加热至90-95℃,然后慢慢加入氯苄。在该温度下反应3小时后冷却,过滤,并将滤液分层,用饱和食盐水洗涤有机层,再用无水硫酸钠干燥。接下来进行减压蒸馏,在81℃(1.6kPa)收集馏分为回收苯胺,在170-190℃(1.6kPa)收集的馏分冷却固化,即得苄基苯胺。精制时可用石油醚重结晶,收率可达80%以上。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-(对氯苄基)苯胺 N-(p-chlorobenzyl)aniline 4750-61-2 C13H12ClN 217.698 对苄氨基苯酚 4-(benzylamino)phenol 103-14-0 C13H13NO 199.252 N,N-甲基苄基苯胺 N-methyl-N-phenyl-benzenemethanamine 614-30-2 C14H15N 197.28 N-苯基-4-甲氧基苄胺 N-(4-methoxybenzyl)aniline 3526-43-0 C14H15NO 213.279 硫苯甲醯胺苯 N-Phenylbenzothioamide 636-04-4 C13H11NS 213.303 N-苄基-N-苯基羟胺 N-benzyl-N-phenylhydroxylamine 3376-40-7 C13H13NO 199.252 N-苄基-2-碘苯胺 N-benzyl-2-iodoaniline 76464-99-8 C13H12IN 309.149 N-(2-碘苯基甲基)苯胺 N-(2-iodobenzyl)aniline 76464-90-9 C13H12IN 309.149 N-苯甲酰替苯胺 N-phenyl benzoyl amide 93-98-1 C13H11NO 197.236 N-苄基-2-氯苯胺 N-benzyl-2-chloroaniline 98018-66-7 C13H12ClN 217.698 Alpha-苄基苯基肼 N-benzyl-N-phenylhydrazine 614-31-3 C13H14N2 198.268 N-苄基-2-溴苯胺 N-benzyl-2-bromoaniline 71687-81-5 C13H12BrN 262.149 N-(2-氯苄基)苯胺 N-(2-chlorobenzyl)aniline 41001-24-5 C13H12ClN 217.698 4-氨基苯甲酰苯胺 N-(4-aminophenyl)benzamide 17625-83-1 C13H12N2O 212.251 甲酰胺,N-苯基-N-(苯基甲基)- N-benzylformanilide 16350-99-5 C14H13NO 211.263 —— N-benzyl-N-phenyl thioformamide 36325-43-6 C14H13NS 227.33 4-氯苯 N-苯甲酰苯胺 4-chlorobenzanilide 6833-15-4 C13H10ClNO 231.681 4-氟-N-苯基苯甲酰胺 4-fluoro-N-phenylbenzamide 366-63-2 C13H10FNO 215.227 苯基-苯基氨基-乙腈 2-anilino-2-phenylacetonitrile 4553-59-7 C14H12N2 208.263 N,N-二苄基-4-氯苯胺 N,N-dibenzyl-4-chloroaniline 15429-21-7 C20H18ClN 307.823 亚硝基苄基苯胺 N-benzyl-N-phenylnitrous amide 612-98-6 C13H12N2O 212.251 —— N-(o-bromophenyl)-o-bromobenzylamine 25261-48-7 C13H11Br2N 341.045 —— N-(2-chlorobenzyl)-2-chloroaniline 88450-73-1 C13H11Cl2N 252.143 —— N-allyl-N-benzylaniline 31930-96-8 C16H17N 223.318 —— N-benzyl-N-(2-propynyl)aniline 2532-70-9 C16H15N 221.302 N-茴香酰胺 N-(p-methoxyphenyl)benzamide 7472-54-0 C14H13NO2 227.263 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N1-苄基苯-1,4-二胺 N1-benzylbenzene-1,4-diamine 17272-83-2 C13H14N2 198.268 苄基对甲苯胺 N-Benzyl-4-methylaniline 5405-15-2 C14H15N 197.28 N-(4-氯-苯基)-苯甲酰胺 N-(4-chlorophenyl)benzylamine 2948-37-0 C13H12ClN 217.698 N-(4-溴苯基)-苯甲酰胺 N-benzyl-N-(4-bromophenyl)amine 2879-83-6 C13H12BrN 262.149 —— p-(benzylamino)benzaldehyde 518071-13-1 C14H13NO 211.263 N1-苄基苯-1,2-二胺 N-benzyl-1,2-phenylenediamine 5822-13-9 C13H14N2 198.268 —— 4-chloro-N-(4-chlorobenzyl)aniline 13159-74-5 C13H11Cl2N 252.143 N,N-甲基苄基苯胺 N-methyl-N-phenyl-benzenemethanamine 614-30-2 C14H15N 197.28 —— N,N'-dibenzylaminodiphenylmethane 51050-64-7 C27H26N2 378.517 —— 4-Benzalaminoazobenzol 10282-37-8 C19H17N3 287.364 N-苄基-4-甲氧基苯胺 N-benzyl-p-anisidine 17377-95-6 C14H15NO 213.279 N-(1-苯基乙基)苯胺 phenyl(1-phenylethyl)amine 779-54-4 C14H15N 197.28 硫苯甲醯胺苯 N-Phenylbenzothioamide 636-04-4 C13H11NS 213.303 —— N-benzyl-N-bromo-aniline —— C13H12BrN 262.149 N-苄基-N-苯基羟胺 N-benzyl-N-phenylhydroxylamine 3376-40-7 C13H13NO 199.252 —— o-(benzylamino)phenol 46425-95-0 C13H13NO 199.252 N-苯甲酰替苯胺 N-phenyl benzoyl amide 93-98-1 C13H11NO 197.236 Alpha-苄基苯基肼 N-benzyl-N-phenylhydrazine 614-31-3 C13H14N2 198.268 —— α-deuterio-N-phenylbenzylamine 57183-82-1 C13H13N 184.245 N-苯甲基-4-硝基苯胺 N-benzyl-4-nitroaniline 14309-92-3 C13H12N2O2 228.25 —— 4-Benzylamino-diphenylselenid 62336-68-9 C19H17NSe 338.311 —— N-(4-nitrobenzyl)aniline 10359-18-9 C13H12N2O2 228.25 N,N-二苄基苯胺 N,N-dibenzylaniline 91-73-6 C20H19N 273.378 N,N-二苯基苄胺 N,N-diphenylbenzylamine 606-87-1 C19H17N 259.351 —— N-(diphenylmethyl)aniline 1865-12-9 C19H17N 259.351 —— N-benzyl-N-(4-methylbenzyl)aniline 111978-56-4 C21H21N 287.404 —— N-Benzyl-4-[(E)-(4-chlorophenyl)diazenyl]aniline 110320-99-5 C19H16ClN3 321.809 N-乙基-N-苄基苯胺 N-benzyl-N-ethylaniline 92-59-1 C15H17N 211.307 2-(苄基氨基)苄腈 2-(benzylamino)benzonitrile 5589-62-8 C14H12N2 208.263 —— N-Benzyl-anilinoacetylen 38488-71-0 C15H13N 207.275 甲酰胺,N-苯基-N-(苯基甲基)- N-benzylformanilide 16350-99-5 C14H13NO 211.263 N-苄基-2-氨基苯乙烯 N-benzyl-2-vinylaniline 210536-20-2 C15H15N 209.291 —— N-benzyl-2,4-dibromoaniline 81090-49-5 C13H11Br2N 341.045 N-(1-苯基丙基)苯胺 N-(1-phenylpropyl)aniline 22920-59-8 C15H17N 211.307 —— N-Benzyl-2,4,6-trichloroaniline 158019-18-2 C13H10Cl3N 286.588 —— N-(1-phenylallyl)aniline 35755-81-8 C15H15N 209.291 苯基-苯基氨基-乙腈 2-anilino-2-phenylacetonitrile 4553-59-7 C14H12N2 208.263 —— N-(phenylmethyl-d2)aniline 136295-03-9 C13H13N 185.237 N,N-联苄基-4-溴苯胺 N,N-dibenzyl-4-bromoaniline 65145-14-4 C20H18BrN 352.274 —— N-(3-chloro-4-fluorophenyl)-benzenemethanamine 131776-32-4 C13H11ClFN 235.688 亚硝基苄基苯胺 N-benzyl-N-phenylnitrous amide 612-98-6 C13H12N2O 212.251 N-苄基-2-(2-丙烯基)苯胺 N-benzyl-2-allylaniline 150596-82-0 C16H17N 223.318 —— N-(3-nitrobenzyl)aniline 3430-70-4 C13H12N2O2 228.25 2-[苄基(苯基)氨基]-乙醇 2-(N-benzyl-N-phenylamino)ethanol 33905-47-4 C15H17NO 227.306 (苄基苯基氨基)乙腈 2-(benzyl(phenyl)amino)acetonitrile 36271-19-9 C15H14N2 222.29 —— N-benzyl-N-(2-chloroethyl)aniline —— C15H16ClN 245.752 —— p-(N-Benzylanilino)-phenol 31310-73-3 C19H17NO 275.35 —— N-allyl-N-benzylaniline 31930-96-8 C16H17N 223.318 —— N-benzyl-N-(2-propynyl)aniline 2532-70-9 C16H15N 221.302 —— N-(2-methyl-1-phenylpropyl)aniline 145909-49-5 C16H19N 225.334 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:叔丁基亚硝酸盐在温和条件下对N-烷基苯胺的区域选择性硝化摘要:据报道使用亚硝酸叔丁酯对N-烷基苯胺的区域选择性环硝化。该反应在多种底物下有效地进行,以优异的产率提供了合成上有用的N-亚硝基N-烷基硝基苯胺,可以分别使用Zn-AcOH和HCl / MeOH轻松地将其转化为N-烷基苯二胺和N-烷基硝基苯胺。DOI:10.1021/acs.joc.8b02377
-
作为产物:参考文献:名称:三氯化 钛(iii)的水溶液将肼还原为胺†摘要:肼中N–N键的裂解被广泛用于胺的制备中,因此在有机合成中占有重要地位。在本文中,我们报告了一种新的方法,该方法可通过市售廉价的水溶液还原肼中的N–N键三氯化钛。反应可在从酸性到中性和碱性的宽pH范围内顺利进行,从而以良好的收率得到胺。此方法与包含官能团的底物兼容,例如,CC双键,苄基-氮键,苄氧基和酰基。DOI:10.1039/c1ob05328k
-
作为试剂:描述:对甲苯硫酰苯胺 在 苯甲基苯胺 、 sodium hydride 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 、 mineral oil 为溶剂, 反应 1.0h, 生成 N-苯基氨基乙酸乙基醚 、 对甲苯亚磺酸参考文献:名称:通过中性有机供体的光活化电子转移对 C?N 和 S?N 键进行无金属还原裂解摘要:光活化的中性有机超电子供体在室温下裂解衍生自二烷基胺的具有挑战性的芳烃磺酰胺。它还裂解 a) ArC NR 和 b) ArN C 键。该研究还强调了通过降低 LUMO 能量和稳定碎裂产物,连接到 (a) 中的 N 和连接到 (b) 中的 C 的基团对这些裂解反应的帮助。DOI:10.1002/anie.201306543
文献信息
-
[EN] THIOPHENE DERIVATIVES FOR THE TREATMENT OF DISORDERS CAUSED BY IGE<br/>[FR] DÉRIVÉS DE THIOPHÈNE POUR LE TRAITEMENT DE TROUBLES PROVOQUÉS PAR IGE申请人:UCB BIOPHARMA SRL公开号:WO2019243550A1公开(公告)日:2019-12-26Thiophene derivatives of formula (I) and a pharmaceutically acceptable salt thereof are provided. These compounds have utility for the treatment or prevention of disorders caused by IgE, such as allergy, type 1 hypersensitivity or familiar sinus inflammation.
-
[EN] SUBSTITUTED BENZYLAMINE COMPOUNDS, THEIR USE IN MEDICINE, AND IN PARTICULAR THE TREATMENT OF HEPATITIS C VIRUS (HCV) INFECTION<br/>[FR] COMPOSÉS DE BENZYLAMINE SUBSTITUÉS, LEUR UTILISATION EN MÉDECINE, EN PARTICULIER DANS LE TRAITEMENT D'UNE INFECTION PAR LE VIRUS DE L'HÉPATITE C (VHC)申请人:ASTEX THERAPEUTICS LTD公开号:WO2013064538A1公开(公告)日:2013-05-10The invention provides compounds of the formula (I): or a salt, N-oxide or tautomer thereof, wherein A is CH, CF or nitrogen; E is CH, CF or nitrogen; and R0 is hydrogen or C1-2 alkyl; R1a is selected from CONH2; CO2H; an optionally substituted acyclic C1-8 hydrocarbon group; and an optionally substituted monocyclic carbocyclic or heterocyclic group of 3 to 7 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S; R2 is selected from hydrogen and a group R2a; R2a is selected from an optionally substituted acyclic d-8 hydrocarbon group; an optionally substituted monocyclic carbocyclic or heterocyclic group of 3 to 7 ring members, of which 0, 1 or 2 ring members are heteroatom ring members selected from O, N and S; and an optionally substituted bicyclic heterocyclic group of 9 or 10 ring members, of which 1 or 2 ring members are nitrogen atoms; wherein at least one of R1 and R2 is other than hydrogen; R3 is an optionally substituted 3- to 10-membered monocyclic or bicyclic carbocyclic or heterocyclic ring containing 0, 1, 2 or 3 heteroatom ring members selected from N, O and S; R4a is selected from halogen; cyano; C1-4 alkyl optionally substituted with one or more fluorine atoms; C1-4 alkoxy optionally substituted with one or more fluorine atoms; hydroxy-C1-4 alkyl; and C1-2 alkoxy-C1-4 alkyl; R5 is selected from hydrogen and a substituent R5a; and R5a is selected from C1-2 alkyl optionally substituted with one or more fluorine atoms; C1-3 alkoxy optionally substituted with one or more fluorine atoms; halogen; cyclopropyl; cyano; and amino, The compounds have activity against hepatitis C virus and can be used in the prevention or treatment of hepatitis C viral infections.该发明提供了以下式(I)的化合物,或其盐、N-氧化物或互变异构体,其中A为CH、CF或氮;E为CH、CF或氮;R0为氢或C1-2烷基;R1a选自CONH2;CO2H;一个可选择取代的非环状C1-8碳氢化合物基团;以及一个可选择取代的含有3至7个环成员的单环碳环或杂环基团,其中0、1、2、3或4个是从O、N和S中选择的杂原子环成员;R2选自氢和一个基团R2a;R2a选自一个可选择取代的非环状d-8碳氢化合物基团;一个可选择取代的含有3至7个环成员的单环碳环或杂环基团,其中0、1或2个环成员是从O、N和S中选择的杂原子环成员;以及一个可选择取代的含有9或10个环成员的双环杂环基团,其中1或2个环成员是氮原子;其中R1和R2中至少一个不是氢;R3选自一个可选择取代的含有0、1、2或3个从N、O和S中选择的杂原子环成员的3至10个成员的单环或双环碳环或杂环环;R4a选自卤素;氰基;C1-4烷基,可选择取代一个或多个氟原子;C1-4烷氧基,可选择取代一个或多个氟原子;羟基-C1-4烷基;和C1-2烷氧基-C1-4烷基;R5选自氢和一个取代基R5a;R5a选自C1-2烷基,可选择取代一个或多个氟原子;C1-3烷氧基,可选择取代一个或多个氟原子;卤素;环丙基;氰基;和氨基。这些化合物对丙型肝炎病毒具有活性,并可用于预防或治疗丙型肝炎病毒感染。
-
The titanocene-catalyzed reduction of acetamides to tertiary amines by PhMeSiH<sub>2</sub>作者:Kumaravel Selvakumar、Kesamreddy Rangareddy、John F HarrodDOI:10.1139/v04-063日期:2004.8.1
A variety of acetamide derivatives are reduced in excellent yields to tertiary amines by PhMeSiH2 in the presence of Cp2TiX2 (X = F or Me) catalysts. The reactions are very clean at 80 °C. At room temperature a secondary reaction, hydrogenolysis of the C(O)N bond, intervenes and reduces the chemoselectivity. Nevertheless, this chemistry provides a simple methodology for the amide/alkylamine transformation using inexpensive, commercially available reagents.Key words: amides, reduction, secondary amides, methylphenylsilane, titanocene, catalysis.
-
Homogeneous Catalytic Hydrogenation of Amides to Amines作者:Jacorien Coetzee、Deborah L. Dodds、Jürgen Klankermayer、Sandra Brosinski、Walter Leitner、Alexandra M. Z. Slawin、David J. Cole-HamiltonDOI:10.1002/chem.201204270日期:2013.8.12Hydrogenation of amides in the presence of [Ru(acac)3] (acacH=2,4‐pentanedione), triphos [1,1,1‐tris‐ (diphenylphosphinomethyl)ethane] and methanesulfonic acid (MSA) produces secondary and tertiary amines with selectivities as high as 93 % provided that there is at least one aromatic ring on N. The system is also active for the synthesis of primary amines. In an attempt to probe the role of MSA and在[Ru(acac)3 ](acacH = 2,4-戊二酮),三[[1,1,1-三(二苯基膦甲基)乙烷]]和甲磺酸(MSA)的存在下进行酰胺加氢生成仲胺和叔胺如果在N上至少有一个芳环,则其选择性高达93%。该系统对伯胺的合成也具有活性。为了探索MSA的作用和反应机理,已经从[Ru(acac)3 ],三醇和MSA或[RuX(OAc)(triphos)]的反应中制备了一系列甲磺酸钠络合物。 (X = H或OAc)或[RuH 2(CO)(triphos )]与MSA。晶体学表征复合物包括:[茹(OAC-κ 1 O)2(H 2O)(triphos)],[Ru(OAc‐κ 2 O,O')(CH 3 SO 3 ‐κ 1 O)(triphos )],[Ru(CH 3 SO 3‐ κ 1 O)2(H 2 O)(三膦)]和[孺2(μ-CH 3 SO 3)3(三磷酸)2 ] [CH 3 SO 3 ],而其他复合物,例如[茹(OAC-κ
-
Cobalt nanoclusters coated with N-doped carbon for chemoselective nitroarene hydrogenation and tandem reactions in water作者:Silvia Gutiérrez-Tarriño、Sergio Rojas-Buzo、Christian W. Lopes、Giovanni Agostini、Jose. J. Calvino、Avelino Corma、Pascual Oña-BurgosDOI:10.1039/d1gc00706h日期:——selective non-noble metal-based catalysts for the chemoselective reduction of nitro compounds in aquo media under mild conditions is an attractive research area. Herein, the synthesis of subnanometric and stable cobalt nanoclusters, covered by N-doped carbon layers as core–shell (Co@NC-800), for the chemoselective reduction of nitroarenes is reported. The Co@NC-800 catalyst was prepared by the pyrolysis用于在温和条件下化学选择性还原水介质中硝基化合物的活性和选择性非贵金属基催化剂的开发是一个有吸引力的研究领域。在此,报道了合成亚纳米和稳定的钴纳米团簇,由 N 掺杂的碳层作为核 - 壳层(Co@NC-800)覆盖,用于硝基芳烃的化学选择性还原。所述钴@ NC-800催化剂是由钴(TPY)的热解制备的2复合浸渍在 Vulcan 碳上。事实上,基于六个 N-Co 键的分子复合物的使用推动了由 N 掺杂碳层覆盖的明确和分布的钴核-壳纳米簇的形成。为了阐明它的性质,它已经通过使用几种先进的技术来充分表征。此外,这种制备的催化剂在温和的反应条件下对用H 2还原硝基化合物显示出高活性、化学选择性和稳定性。水被用作绿色溶剂,改善了之前基于钴催化剂的结果。此外,Co@NC-800通过硝基芳烃的还原胺化,该催化剂对于一锅合成仲芳基胺和异吲哚啉酮也具有活性和选择性。最后,基于衍射和光谱研究,已提出具有表面 CoN
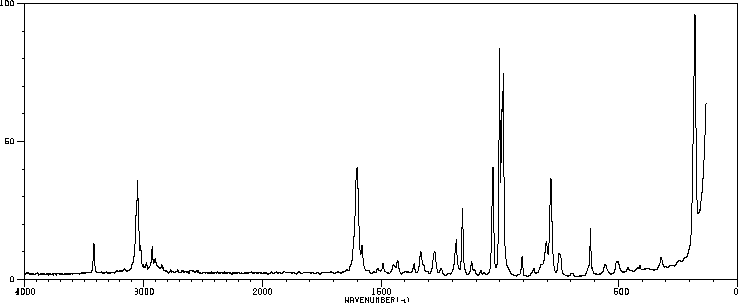
表征谱图
-
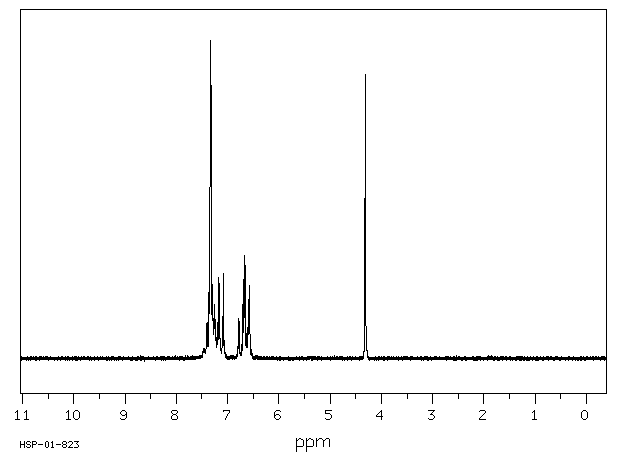
氢谱1HNMR
-
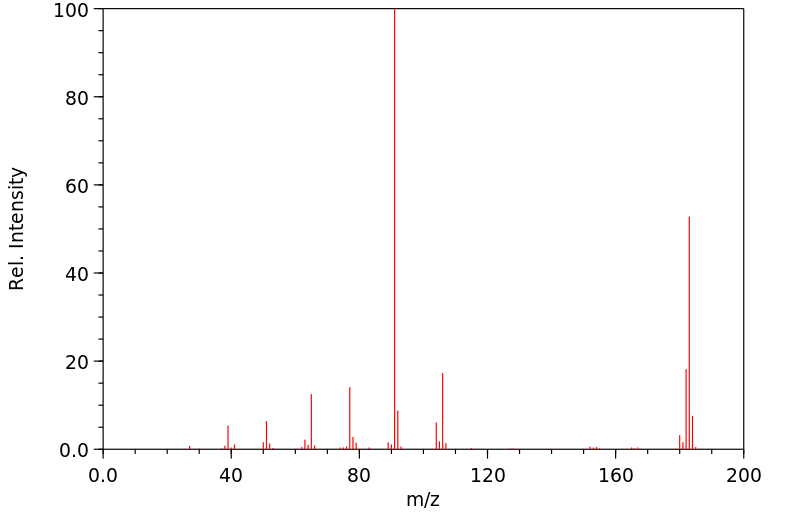
质谱MS
-
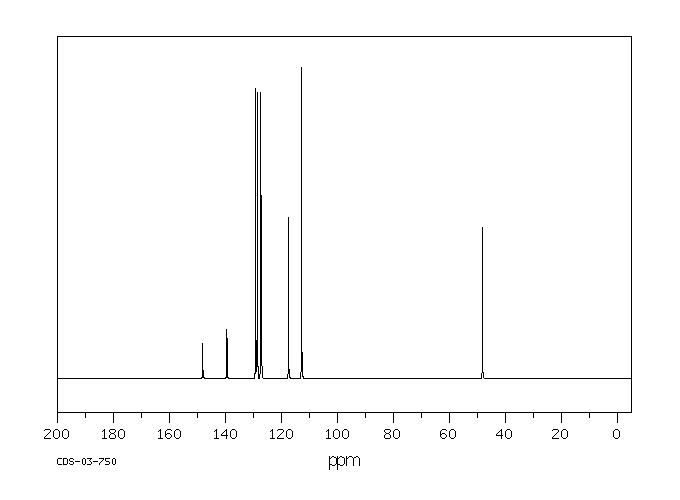
碳谱13CNMR
-
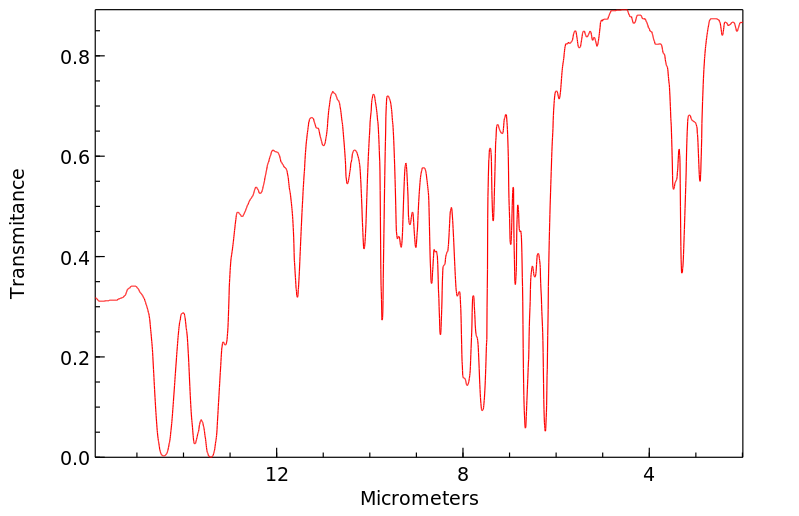
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息