N-苯基-4-甲氧基苄胺 | 3526-43-0
中文名称
N-苯基-4-甲氧基苄胺
中文别名
——
英文名称
N-(4-methoxybenzyl)aniline
英文别名
N-phenyl-(p-methoxybenzyl) amine;N-(4-methoxybenzyl)benzenamine;(4-methoxybenzyl)phenylamine;N-(4-methoxylbenzyl)aniline;N-phenyl-4-methoxybenzylamine;4-methoxy-N-phenylbenzenemethanamine;N-[(4-methoxyphenyl)methyl]aniline;N-(p-methoxybenzyl)aniline;(4-methoxybenzyl)aniline
CAS
3526-43-0
化学式
C14H15NO
mdl
——
分子量
213.279
InChiKey
WRDZMZGYHVUYRU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:16
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:21.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2922299090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:室温且干燥
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4-methoxy-N-phenylthiobenzamide 26060-23-1 C14H13NOS 243.329 N-苯基-4-甲氧基苯甲酰胺 4-methoxy-N-phenylbenzamide 7465-88-5 C14H13NO2 227.263 4-甲氧基苄胺 4-methoxy-benzylamine 2393-23-9 C8H11NO 137.181 N-(2-溴苯基)-4-甲氧基苯甲酰胺 N-(2-bromophenyl)-4-methoxybenzamide 349614-89-7 C14H12BrNO2 306.159 —— N-(4-methoxybenzyl)-N-phenylacetamide 81575-55-5 C16H17NO2 255.316 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-[(4-甲氧基苯基)甲基],N-甲基苯胺 N-(4-methoxybenzyl)-N-methylaniline 37931-52-5 C15H17NO 227.306 N-苯基-4-甲氧基苯甲酰胺 4-methoxy-N-phenylbenzamide 7465-88-5 C14H13NO2 227.263 —— N-benzyl-N-(4-methoxybenzyl)aniline —— C21H21NO 303.404 苯甲基苯胺 N-Benzylaniline 103-32-2 C13H13N 183.253 —— N-[1-(4-methoxyphenyl)-2-propenyl]aniline —— C16H17NO 239.317 2-苯胺基-2-(4-甲氧基苯基)乙腈 2-anilino-2-(p-methoxyphenyl)acetonitrile 15190-69-9 C15H14N2O 238.289 —— N-(1-(4-methoxyphenyl)but-3-enyl)benzenamine —— C17H19NO 253.344 —— N-(4-methoxybenzyl)-N-phenylacetamide 81575-55-5 C16H17NO2 255.316 —— N-(4-methoxyphenylmethyl)-N-phenylcarbamoyl chloride 4678-32-4 C15H14ClNO2 275.735
反应信息
-
作为反应物:描述:参考文献:名称:聚合物包埋的金纳米团簇催化的胺有氧氧化:聚合物中团簇大小和协同官能团的影响摘要:开发了由聚合物包埋的 Au 纳米团簇 (PI-Au) 催化的胺到亚胺的有氧氧化反应。仔细检查了簇大小对这种氧化反应的影响......DOI:10.1246/bcsj.20100300
-
作为产物:描述:N-苯基-4-甲氧基苯甲酰胺 在 0.55C27H43N3Si3V*0.45C27H44N3Si3V 、 频那醇硼烷 作用下, 以 四氢呋喃 为溶剂, 反应 16.0h, 以71%的产率得到N-苯基-4-甲氧基苄胺参考文献:名称:对“用于化学选择性还原C═X(X = O,N)功能的氧化还原非配体负载的钒催化剂”的修正。摘要:页面15232、15233和15236。在原始论文中,在2.3和2.8节中分别错误地报告了2 1和2 1a / 2 1b的双峰波函数被自旋污染(分别为图3和方案9)。从0.75自旋平方操作者⟨的错误地报告预期特征值S ^ 2的反铁磁性耦合双峰⟩|↓⟩大号|↑↑⟩ V状态(最初在支持信息中给出)。↓⟩|为正确的预期特征值大号|↑↑⟩ V状态应该是1.75。2 1和2 1a /的波动函数因此,2 1b(分别为特征值1.79和1.77 / 1.66)没有受到自旋污染。校正后的图3和方案9如下。还提供了正确的支持信息文件。更正不会影响该文章的任何结论,但是会稍微减小四重奏和双重旋曲面之间的间隙。方案3也已得到纠正,以反映以下事实:(CH 3)3 SiCH 2 •自由基只能基于自旋守恒来反应。图3.不同的UDFT / SMD(Et 2 O)功能/基集对1的双重态和四重态自旋态之间的能量差的影响。马利肯自旋密度图(黄,α-自旋;蓝色,βDOI:10.1021/jacs.0c09331
-
作为试剂:描述:N-苯基-4-甲氧基苄胺 、 在 N-苯基-4-甲氧基苄胺 作用下, 以68的产率得到N1-(4-methoxybenzyl)-N4-methyl-N1,N4-diphenylbut-2-ynediamide参考文献:名称:Adv. Synth. Catal. 2016, 358, 3534-3540摘要:DOI:
文献信息
-
The titanocene-catalyzed reduction of acetamides to tertiary amines by PhMeSiH<sub>2</sub>作者:Kumaravel Selvakumar、Kesamreddy Rangareddy、John F HarrodDOI:10.1139/v04-063日期:2004.8.1
A variety of acetamide derivatives are reduced in excellent yields to tertiary amines by PhMeSiH2 in the presence of Cp2TiX2 (X = F or Me) catalysts. The reactions are very clean at 80 °C. At room temperature a secondary reaction, hydrogenolysis of the C(O)N bond, intervenes and reduces the chemoselectivity. Nevertheless, this chemistry provides a simple methodology for the amide/alkylamine transformation using inexpensive, commercially available reagents.Key words: amides, reduction, secondary amides, methylphenylsilane, titanocene, catalysis.
-
Homogeneous Catalytic Hydrogenation of Amides to Amines作者:Jacorien Coetzee、Deborah L. Dodds、Jürgen Klankermayer、Sandra Brosinski、Walter Leitner、Alexandra M. Z. Slawin、David J. Cole-HamiltonDOI:10.1002/chem.201204270日期:2013.8.12Hydrogenation of amides in the presence of [Ru(acac)3] (acacH=2,4‐pentanedione), triphos [1,1,1‐tris‐ (diphenylphosphinomethyl)ethane] and methanesulfonic acid (MSA) produces secondary and tertiary amines with selectivities as high as 93 % provided that there is at least one aromatic ring on N. The system is also active for the synthesis of primary amines. In an attempt to probe the role of MSA and在[Ru(acac)3 ](acacH = 2,4-戊二酮),三[[1,1,1-三(二苯基膦甲基)乙烷]]和甲磺酸(MSA)的存在下进行酰胺加氢生成仲胺和叔胺如果在N上至少有一个芳环,则其选择性高达93%。该系统对伯胺的合成也具有活性。为了探索MSA的作用和反应机理,已经从[Ru(acac)3 ],三醇和MSA或[RuX(OAc)(triphos)]的反应中制备了一系列甲磺酸钠络合物。 (X = H或OAc)或[RuH 2(CO)(triphos )]与MSA。晶体学表征复合物包括:[茹(OAC-κ 1 O)2(H 2O)(triphos)],[Ru(OAc‐κ 2 O,O')(CH 3 SO 3 ‐κ 1 O)(triphos )],[Ru(CH 3 SO 3‐ κ 1 O)2(H 2 O)(三膦)]和[孺2(μ-CH 3 SO 3)3(三磷酸)2 ] [CH 3 SO 3 ],而其他复合物,例如[茹(OAC-κ
-
Main-Group-Catalyzed Reductive Alkylation of Multiply Substituted Amines with Aldehydes Using H<sub>2</sub>作者:Yoichi Hoshimoto、Takuya Kinoshita、Sunit Hazra、Masato Ohashi、Sensuke OgoshiDOI:10.1021/jacs.8b03626日期:2018.6.13the growing demand for green and sustainable chemical processes, the catalytic reductive alkylation of amines with main-group catalysts of low toxicity and molecular hydrogen as the reductant would be an ideal method to functionalize amines. However, such a process remains challenging. Herein, a novel reductive alkylation system using H2 is presented, which proceeds via a tandem reaction that involves鉴于对绿色和可持续化学过程的需求不断增长,以低毒主族催化剂和分子氢为还原剂的胺的催化还原烷基化将是一种理想的胺官能化方法。然而,这样的过程仍然具有挑战性。在此,提出了一种使用 H2 的新型还原性烷基化系统,该系统通过串联反应进行,该反应涉及 B(2,6-Cl2C6H3)(p-HC6F4)2 催化形成亚胺,随后通过沮丧的刘易斯对(FLP)。这种还原性烷基化反应产生 H2O 作为唯一的副产物,并直接将带有广泛取代基的胺官能化,这些取代基包括羧基、羟基、附加氨基、伯酰胺和伯磺酰胺基团。异吲哚啉酮和氨基邻苯二甲酸酐的合成也已通过一锅法实现,该法分别由本发明的还原烷基化与分子内酰胺化和分子内脱水反应的组合组成。该反应分别显示出对亚胺中间体和 B(2,6-Cl2C6H3)(p-HC6F4)2 浓度的零级和一级依赖性。此外,H2 浓度显着影响反应进程。这些结果表明了一种可能的机制,其中包含 THF 和 B(2
-
Direct One‐Pot Reductive N‐Alkylation of Nitroarenes by using Alcohols with Supported Gold Catalysts作者:Chun‐Hong Tang、Lin He、Yong‐Mei Liu、Yong Cao、He‐Yong He、Kang‐Nian FanDOI:10.1002/chem.201100393日期:2011.6.20Gold standard support! The direct reductive mono‐ or di‐N‐alkylation of aromatic nitro compounds with alcohols, using a hydrogen‐borrowing strategy, was efficiently promoted by a ligand‐free titania‐supported gold catalyst system (see scheme). A variety of nitroarenes were selectively converted into the corresponding secondary or tertiary amines in good to excellent yields without any co‐catalysts
-
Green synthesis and catalytic properties of palladium nanoparticles for the direct reductive amination of aldehydes and hydrogenation of unsaturated ketones作者:Mahmoud NasrollahzadehDOI:10.1039/c4nj01440e日期:——This paper reports on the synthesis and use of palladium nanoparticles as heterogeneous catalysts for the reductive amination of aldehydes and hydrogenation of unsaturated ketones. This method has the advantages of high yields, simple methodology and easy work up. The catalyst can be recovered and reused several times without significant loss of catalytic activity.本文报道了钯纳米粒子的合成及其作为非均相催化剂用于醛的还原胺化和不饱和酮加氢的应用。该方法具有收率高,方法简单,后处理容易的优点。催化剂可以回收并重复使用几次,而不会显着降低催化活性。
表征谱图
-
氢谱1HNMR
-
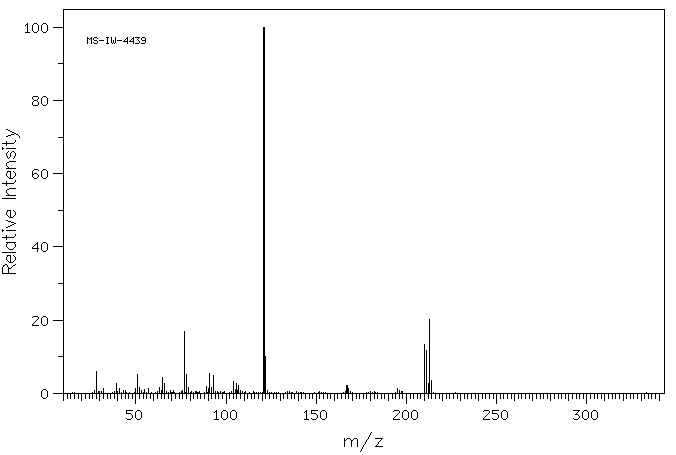
质谱MS
-
碳谱13CNMR
-
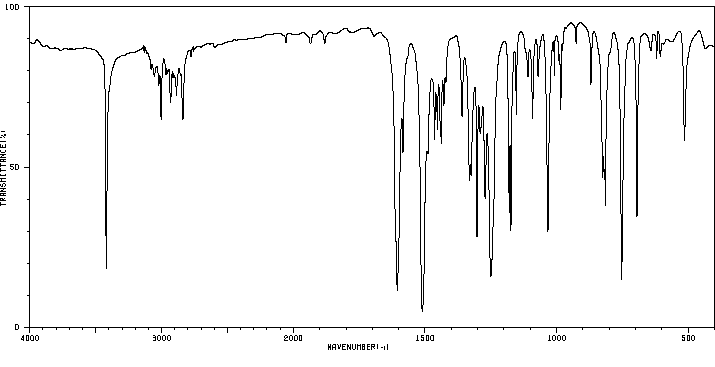
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫