4-氨基苯甲酰苯胺 | 17625-83-1
中文名称
4-氨基苯甲酰苯胺
中文别名
4-氨基苯酰苯胺;红色基DB-90;对氨基苯甲酰苯胺;4-氨基苯甲酰替苯胺;4′-苯甲酰胺基苯胺;对氨基苯酰替苯胺;4-氨基萘氨;4'-氨基苯甲酰苯胺;4-苯甲酰胺基苯胺
英文名称
N-(4-aminophenyl)benzamide
英文别名
4’-aminobenzanilide;4'-Aminobenzanilide
CAS
17625-83-1
化学式
C13H12N2O
mdl
MFCD00035777
分子量
212.251
InChiKey
GTTFJYUWPUKXJH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:127-131 °C(lit.)
-
沸点:308.6±25.0 °C(Predicted)
-
密度:1.244±0.06 g/cm3(Predicted)
-
溶解度:溶于二甲基亚砜和甲醇。
-
稳定性/保质期:
常温常压下稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:55.1
-
氢给体数:2
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2924299090
-
危险类别:IRRITANT
-
安全说明:S26,S36
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:常温下应密闭避光保存,并保持通风和干燥。
SDS
| Name: | N-Benzoylphenylenediamine 98% Material Safety Data Sheet |
| Synonym: | 1-Amino-4-benzoylaminobenzen |
| CAS: | 17625-83-1 |
Synonym:1-Amino-4-benzoylaminobenzen
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 17625-83-1 | N-Benzoylphenylenediamine | 98% | 241-603-0 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 17625-83-1: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C13H12N2O
Molecular Weight: 212.25
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, acids, acetic anhydride, acid chlorides, carbon dioxide.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 17625-83-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
N-Benzoylphenylenediamine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 17625-83-1: 2
Canada
CAS# 17625-83-1 is listed on Canada's NDSL List.
CAS# 17625-83-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 17625-83-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苯甲酰替苯胺 N-phenyl benzoyl amide 93-98-1 C13H11NO 197.236 —— N-(4’-acetamidophenyl)benzamide 331240-43-8 C15H14N2O2 254.288 4'-硝基苯甲酰苯胺 p-nitrobenzanilide 3393-96-2 C13H10N2O3 242.234 —— N,N-diphenylbenzamide 4051-56-3 C19H15NO 273.334 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-(4-(methylamino)phenyl)benzamide 55872-58-7 C14H14N2O 226.278 N,N'-(对亚苯基)二苯甲酰胺 N,N'-benzene-1,4-diyldibenzamide 5467-04-9 C20H16N2O2 316.359 —— N-[4-(ethylamino)phenyl]benzamide —— C15H16N2O 240.305 —— N-(4-(phenylamino)phenyl)benzamide 23058-58-4 C19H16N2O 288.349 —— N-(4-azidophenyl)benzamide —— C13H10N4O 238.249 —— N-(4-benzamidophenyl)-4-fluorobenzamide —— C20H15FN2O2 334.35 —— (E)-N,N'-(diazene-1,2-diylbis(4,1-phenylene))dibenzamide —— C26H20N4O2 420.47 —— N1,N3-bis(4-benzamidophenyl)benzene-1,3-dicarboxamide 68898-65-7 C34H26N4O4 554.605 —— N1,N3,N5-tris(4-benzamidophenyl)benzene-1,3,5-tricarboxamide 1422521-32-1 C48H36N6O6 792.85 —— N-[4-(3,5-dihydroxybenzylamino)phenyl]benzamide 1022159-98-3 C20H18N2O3 334.375 —— N-(4-benzamidophenyl)-2-hydroxybenzamide —— C20H16N2O3 332.359 —— N-(4-benzamidophenyl)-2-chbrobenzamide 300670-37-5 C20H15ClN2O2 350.804 5-{[4-(苯甲酰基氨基)苯基]氨基}-5-氧代戊酸 5-<<4-(benzoylamino)phenyl>amino>-5-oxopentanoic acid 134485-49-7 C18H18N2O4 326.352 —— N-[4-(3-tetradecylureido)phenyl]benzamide —— C28H41N3O2 451.652 N1-苄基苯-1,4-二胺 N1-benzylbenzene-1,4-diamine 17272-83-2 C13H14N2 198.268 —— N-(4-benzamidophenyl)furan-2-carboxamide —— C18H14N2O3 306.3 —— N-[4-(propan-2-ylsulfonylamino)phenyl]benzamide 1092521-91-9 C16H18N2O3S 318.397 苯甲基苯胺 N-Benzylaniline 103-32-2 C13H13N 183.253 —— N-(4-(4H-1,2,4-triazol-4-yl)phenyl)benzamide —— C15H12N4O 264.286 —— 1-benzoylamino-4-(3-hydroxy-[2]naphthoylamino)-benzene 24363-05-1 C24H18N2O3 382.419 —— N-(4-benzoylamino)phenyl-4-hydroxy-3-methoxybenzamide —— C21H18N2O4 362.385 —— N-(4-(2-carbamoylphenylamino)phenyl)benzamide 1382353-98-1 C20H17N3O2 331.374 —— N4-Benzoyl-N1-(phenylsulfonyl)-1,4-diaminobenzene 327060-18-4 C19H16N2O3S 352.414 —— 4'-[2,3-di(tert-butoxycarbonyl)guanidino]benzanilide 1015690-86-4 C24H30N4O5 454.526 - 1
- 2
- 3
反应信息
-
作为反应物:描述:4-氨基苯甲酰苯胺 在 盐酸 、 sodium nitrite 、 sodium azide 、 sodium acetate 作用下, 以 水 为溶剂, 反应 1.67h, 生成 N-(4-azidophenyl)benzamide参考文献:名称:α-降钙素衍生的乙酰基磺酸三唑衍生物的合成及其对T细胞和B细胞增殖的生物评价摘要:使用Huisgen 1,3-偶极环加成反应(点击化学方法)合成了一系列新的α-桑顿酸衍生的乙酰基桑地酸1,2,3-三唑衍生物,并评估了其对伴刀豆球蛋白A(ConA)的体外抑制活性。 )诱导T细胞增殖,而脂多糖(LPS)诱导B细胞增殖。在合成的系列中,化合物2-10和19对ConA和LPS刺激的T细胞和B细胞增殖呈剂量依赖性显着抑制作用。更重要的是化合物4,9-10和19浓度为1μM时,对有丝分裂原诱导的T细胞和B细胞增殖具有较强的抑制活性,且细胞毒性显着降低。化合物6在100μM浓度下对LPS诱导的B细胞具有约89%的有效免疫抑制作用,对ConA刺激的T细胞增殖有约83%的抑制作用,而无细胞毒性。化合物10对B细胞的增殖更具选择性,在100和1μM的浓度下分别表现出81%和69%的抑制作用。本研究导致鉴定出几种具有降低的细胞毒性和对有丝分裂原诱导的细胞增殖具有强抑制活性的桑托宁类似物。DOI:10.1016/j.ejmech.2016.05.018
-
作为产物:描述:参考文献:名称:三氟化硼-甲醇络合物。温和而强大的试剂,可用于脱保护乙酰基胺。范围和选择性摘要:三氟化硼-甲醇络合物在对乙酰苯胺的脱乙酰作用中对常用的氨基保护基团表现出显着的脱保护选择性,并对底物的空间位阻高度敏感。探索了反应的范围和局限性。DOI:10.1016/j.tetlet.2015.12.087
文献信息
-
Cyclic (Alkyl)(amino)carbene Ligand-Promoted Nitro Deoxygenative Hydroboration with Chromium Catalysis: Scope, Mechanism, and Applications作者:Lixing Zhao、Chenyang Hu、Xuefeng Cong、Gongda Deng、Liu Leo Liu、Meiming Luo、Xiaoming ZengDOI:10.1021/jacs.0c12318日期:2021.1.27Transition metal catalysis that utilizes N-heterocyclic carbenes as noninnocent ligands in promoting transformations has not been well studied. We report here a cyclic (alkyl)(amino)carbene (CAAC) ligand-promoted nitro deoxygenative hydroboration with cost-effective chromium catalysis. Using 1 mol % of CAAC-Cr precatalyst, the addition of HBpin to nitro scaffolds leads to deoxygenation, allowing for利用 N-杂环卡宾作为非无害配体促进转化的过渡金属催化尚未得到很好的研究。我们在这里报告了具有成本效益的铬催化的环状(烷基)(氨基)卡宾(CAAC)配体促进的硝基脱氧硼氢化反应。使用 1 mol % 的 CAAC-Cr 预催化剂,将 HBpin 添加到硝基支架上会导致脱氧,从而保留各种可还原的官能团和敏感基团对硼氢化的相容性,从而提供一种温和、化学选择性和易于形成的策略苯胺,以及杂芳基和脂肪胺衍生物,具有广泛的范围和特别高的转换数(高达 1.8 × 106)。基于理论计算的机械研究,表明CAAC配体在促进HBpin氢化物极性反转中起重要作用;它用作 H 穿梭以促进脱氧硼氢化。通过这种策略制备的几种市售药物突出了其在药物化学中的潜在应用。
-
Alkyne–azide cycloaddition analogues of dehydrozingerone as potential anti-prostate cancer inhibitors <i>via</i> the PI3K/Akt/NF-kB pathway作者:Chetan Kumar、Reyaz Ur Rasool、Zainab Iqra、Yedukondalu Nalli、Prabhu Dutt、Naresh K. Satti、Neha Sharma、Sumit G. Gandhi、Anindya Goswami、Asif AliDOI:10.1039/c7md00267j日期:——
Alkyne–azide cycloaddition derivatives of DHZ (
1 ) were synthesized and screened for cytotoxic potential in which the derivatives,3 ,6 ,7 ,8 ,9 and15 displayed most potent with IC50 value ranging from 1.8–3.0 μM. -
Hydroxy Group Directed Catalytic Hydrosilylation of Amides作者:Jizhi Ni、Tsubasa Oguro、Taka Sawazaki、Youhei Sohma、Motomu KanaiDOI:10.1021/acs.orglett.8b03014日期:2018.12.7Chemo- and site-selective hydrosilylation of α- or β-hydroxy amides using organocatalyst B(C6F5)3 and commercially available hydrosilanes is described. This transformation is operative under mild conditions and tolerates a wide range of functional groups. The reaction was applied for selective reduction of a specific amide group of the therapeutically important cyclic peptide cyclosporin A, demonstrating
-
[EN] PROTEIN KINASE INHIBITORS AND USE THEREOF<br/>[FR] INHIBITEURS DE PROTÉINE KINASE ET LEUR UTILISATION申请人:MERCK SERONO SA公开号:WO2009108670A1公开(公告)日:2009-09-03Disclosed are benzonaphthyridinyl derivative compounds and analogs thereof, pharmaceutical compositions comprising such compounds and processes for preparing the same. The compounds are useful in the treatment of diseases amenable to kinase signal transduction inhibition, regulation or modulation.
-
Improved and General Manganese‐Catalyzed N‐Methylation of Aromatic Amines Using Methanol作者:Jacob Neumann、Saravanakumar Elangovan、Anke Spannenberg、Kathrin Junge、Matthias BellerDOI:10.1002/chem.201605218日期:2017.4.24A novel lutidine‐based manganese PNP‐pincer complex has been synthesized for the selective N‐methylation of aromatic amines with methanol. Using borrowing hydrogen methodology, a selection of differently functionalized aniline derivatives is selectively methylated in good yields.
表征谱图
-
氢谱1HNMR
-
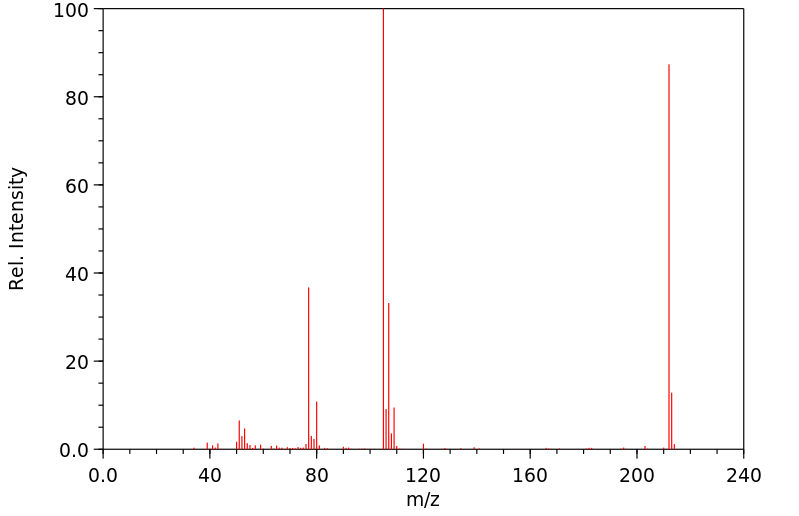
质谱MS
-
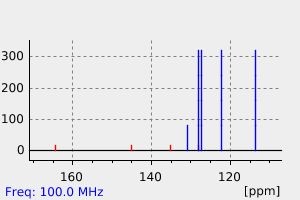
碳谱13CNMR
-
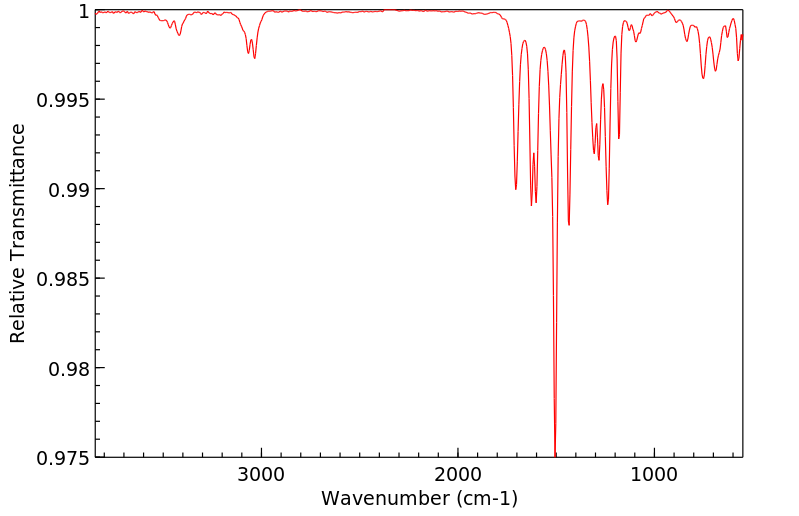
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫