N-乙基-N-苄基苯胺 | 92-59-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:34-36°C
-
沸点:163-164 °C6 mm Hg(lit.)
-
密度:1.029 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
溶解度:酒精:1ml溶于5.5ml
-
LogP:4.38 at 25℃
-
物理描述:N-ethyl-n-benzylaniline appears as a colorless to light colored liquid. Insoluble in water and denser than water. Hence sinks in water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption.
-
颜色/状态:LIGHT YELLOW, OILY LIQUID
-
蒸汽密度:7.28 (NTP, 1992) (Relative to Air)
-
蒸汽压力:Vapor pressure, Pa at 20 °C: 129
-
自燃温度:IGNITION TEMPERATURE BELOW 500 °C (900 °F).
-
分解:313 °C
-
折光率:INDEX OF REFRACTION: 1.5938 AT 23 °C/D
-
保留指数:1727.8
-
稳定性/保质期:
稳定,具有可燃性,不可与强氧化剂、酸类接触。
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:16
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S36/37
-
危险类别码:R20/21/22
-
WGK Germany:2
-
海关编码:2921420090
-
危险品运输编号:UN 2274 6.1/PG 3
-
包装等级:III
-
危险类别:6.1
-
储存条件:本品应密封存于阴凉避光处保存。
SDS
模块 1. 化学品
1.1 产品标识符
: N-苄基-N-乙基苯胺
产品名称
1.2 鉴别的其他方法
N-Ethyl-N-phenylbenzyLAmine
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
急性毒性, 经皮 (类别4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H312 皮肤接触有害。
警告申明
预防
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P280 穿戴防护手套/ 防护服。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P312 如感觉不适,呼救中毒控制中心或医生.
P322 具体措施(见本标签上提供的急救指导)。
P330 漱口。
P363 沾染的衣服清洗后方可重新使用。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: N-Ethyl-N-phenylbenzyLAmine
别名
: C15H17N
分子式
: 211.3 g/mol
分子量
组分 浓度或浓度范围
N-Benzyl-N-ethyLAniline
-
CAS 号 92-59-1
EC-编号 202-169-8
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用惰性吸附材料吸收并当作危险废品处理。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 深黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
163 - 164 °C 在 8 hPa - lit.
g) 闪点
157 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
1.029 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤被吸收是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 2274 国际海运危规: 2274 国际空运危规: 2274
14.2 联合国(UN)规定的名称
欧洲陆运危规: N-ETHYL-N-BENZYLANILINE
国际海运危规: N-ETHYL-N-BENZYLANILINE
国际空运危规: N-Ethyl-N-benzyLAniline
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
化学性质
这是一种浅黄色的油状液体。其熔点为34-36℃,沸点在94.7kPa下约为285-286℃(轻微分解),而在0.8kPa下的沸点是163-164℃,相对密度为1.034(19/4℃),折光率为1.5930。它能够溶解于乙醇及其他有机溶剂,但不溶于水。
用途
该物质用作染料中间体,主要用于制造酸性淡绿SF以及其他蓝色染料。此外,还可以用于生产酸性橙50、红119、蓝5、7和绿5、15、65等染料的中间体。
生产方法
此化合物可由乙基苯胺与氯化苄反应制得。具体操作是在衬铅釜中加入乙基苯胺,边冷却边滴加氯化苄,搅拌10小时后在100℃下保温12小时。使用氢氧化钠溶液洗涤并经过真空蒸馏即可得到成品。另一种方法是以二乙基苯胺为原料,与氯化苄按摩尔比2:1混合,在150℃下加入1%的碘催化反应20小时后,再进行真空蒸馏获得产品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-苄基-N-苯基乙酰胺 N-phenyl-N-benzylacetamide 6840-29-5 C15H15NO 225.29 N-乙基苯甲酰苯胺 N-ethyl-N-phenylbenzamide 16466-44-7 C15H15NO 225.29 2-(苄基(乙基)氨基)苯甲醛 2-(benzyl(ethyl)amino)benzaldehyde 1037132-05-0 C16H17NO 239.317 苯甲基苯胺 N-Benzylaniline 103-32-2 C13H13N 183.253 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-ethyl-N-benzyl-p-phenylenediamine 58979-09-2 C15H18N2 226.321 —— N-benzyl-4-bromo-N-ethylaniline 330793-06-1 C15H16BrN 290.203 —— N-ethyl-N-benzyl-4-nitroso-aniline 3421-02-1 C15H16N2O 240.305 N-乙基-N-苄基-4-氨基苯甲醛 4-N-benzylamino-N-ethylaminobenzaldehyde 67676-47-5 C16H17NO 239.317 —— 4,4'-methylenebis(N-benzyl-N-ethylaniline) 87618-12-0 C31H34N2 434.624 —— N-(p-nitrobenzyl)-N-ethylaniline 37043-80-4 C15H16N2O2 256.304 硫代氰基酸,4-[乙基(苯基甲基)氨基]苯基酯 N-ethyl-N-benzyl-4-thiocyanato-aniline 5335-85-3 C16H16N2S 268.382 —— bis-[4-(ethyl-benzyl-amino)-phenyl]-phenyl-methane 13319-50-1 C37H38N2 510.722 N-乙基苯甲酰苯胺 N-ethyl-N-phenylbenzamide 16466-44-7 C15H15NO 225.29 N-乙基-4-(4-硝基苯基)偶氮-N-(苯基甲基)苯胺 4-<4-Nitro-phenylazo>-N-aethyl-N-benzyl-anilin 3025-78-3 C21H20N4O2 360.415 N-乙基-N(3’-磺酸基)苄基苯胺 4-(N-ethyl-anilinomethyl)-benzenesulfonic acid 92-60-4 C15H17NO3S 291.371 4-(苄基乙基氨基)苯磺酸 N-ethyl-N-benzyl-sulfanilic acid 92-56-8 C15H17NO3S 291.371 —— 1-(4-(4-(N-benzyl-N-ethylamino)phenylazo)phenyl)ethanone 1357026-81-3 C23H23N3O 357.455 —— 2-(N-ethyl(phenyl)amino)-2-phenylacetonitrile 306991-88-8 C16H16N2 236.316 N-乙基-N-[4-(1H-1,2,4-三唑-3-基偶氮)苯基]苄胺 Benzenemethanamine, N-ethyl-N-[4-(1H-1,2,4-triazol-3-ylazo)phenyl]- 13486-13-0 C17H18N6 306.37 3-[(N-乙基苯胺基)甲基]苯磺酰胺 3-(N-ethyl-anilinomethyl)-benzenesulfonic acid amide 56919-72-3 C15H18N2O2S 290.386 N-乙基-N-苄基苯胺-3’-磺酸 N-ethyl-N-benzylaniline-3'-sulfonic acid 101-11-1 C15H17NO3S 291.371 —— methyl 2-(4-(benzyl(ethyl)amino)phenyl)-2-phenylacetate 1262227-18-8 C24H25NO2 359.468 —— 2-[4-(benzyl-ethyl-amino)-phenyl]-1,1,1,3,3,3-hexafluoro-propan-2-ol 872690-44-3 C18H17F6NO 377.329 —— N-benzyl-N-ethyl-4-[(5-methyl-1,2-oxazol-3-yl)diazenyl]aniline 1227044-25-8 C19H20N4O 320.394 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:水滑石中的Mn(III)活性位有效催化分子氧与烷基芳烃的氧化摘要:迫切需要开发基于容易获得的材料和分子氧的高效多相催化体系,以选择性氧化烷基芳烃。在本研究中,已发现NiMn水滑石(Ni 2 Mn-LDH)作为使用分子氧作为唯一氧化剂而不添加任何添加剂的烷基芳烃氧化的有效催化剂。已经观察到令人印象深刻的催化性能,优异的稳定性和可回收性,广泛的适用范围和催化系统的实际潜力。有人认为Mn 3+是有效的活性位点,而Ni 2+在稳定Mn 3+方面起着重要作用。水滑石结构中的物种。动力学研究表明,二苯基甲烷的好氧氧化是Ni 2 Mn-LDH上的一级反应,活化能(E a)和预指数因子(A 0)为85.7 kJ mol -1和1.8×10 9 min -1。吉布斯自由能(ΔG ≠)被确定为-10.4 kJ mol -1 K -1根据Eyring-Polanyi方程的氧化反应,表明该反应是强力的。机理研究表明反应是通过自由基和碳正离子中间体进行的。然后将这两种物质分别俘获分子氧和HDOI:10.1016/j.mcat.2020.111276
-
作为产物:描述:参考文献:名称:由硝基化合物,硼酸和亚磷酸三烷基酯合成无过渡金属的三芳基胺的三组分摘要:芳族胺的合成在化学上一直受到关注。已经实现了一种极其通用的三组分反应,该反应可将廉价的硝基化合物,硼酸和亚磷酸三烷基酯直接转化为叔芳族胺。该反应容许硝基和硼酸部分上的烷基和芳基取代基以及官能化的亚磷酸酯。不需要过渡金属催化。该方法与其他经典的金属催化合成方法正交,因为它可以容忍卤素的存在,并且还可以合成功能化的化合物,例如α-氨基酯衍生物。DOI:10.1002/adsc.201901009
-
作为试剂:描述:参考文献:名称:Manufacture of vinyl ethers摘要:公开号:US02404700A1
文献信息
-
[EN] NOVEL COMPOUNDS, THEIR PREPARATION AND USE<br/>[FR] NOUVEAUX COMPOSES, LEUR PREPARATION ET LEUR UTILISATION申请人:NOVO NORDISK AS公开号:WO2005105736A1公开(公告)日:2005-11-10Novel compounds of the general formula (I), the use of these compounds as phar- maceutical compositions, pharmaceutical compositions comprising the compounds and methods of treatment employing these compounds and compositions. The present compounds may be useful in the treatment and/or prevention of conditions mediated by Peroxisome Proliferator-Activated Receptors (PPAR), in particular the PPARδ suptype.
-
The titanocene-catalyzed reduction of acetamides to tertiary amines by PhMeSiH<sub>2</sub>作者:Kumaravel Selvakumar、Kesamreddy Rangareddy、John F HarrodDOI:10.1139/v04-063日期:2004.8.1
A variety of acetamide derivatives are reduced in excellent yields to tertiary amines by PhMeSiH2 in the presence of Cp2TiX2 (X = F or Me) catalysts. The reactions are very clean at 80 °C. At room temperature a secondary reaction, hydrogenolysis of the C(O)N bond, intervenes and reduces the chemoselectivity. Nevertheless, this chemistry provides a simple methodology for the amide/alkylamine transformation using inexpensive, commercially available reagents.Key words: amides, reduction, secondary amides, methylphenylsilane, titanocene, catalysis.
-
Compounds and uses thereof for decreasing activity of hormone-sensitive lipase申请人:——公开号:US20030166644A1公开(公告)日:2003-09-04Use of compounds to inhibit hormone-sensitive lipase, pharmaceutical compositions comprising the compounds, methods of treatment employing these compounds and compositions, and novel compounds. The present compounds are inhibitors of hormone-sensitive lipase and may be useful in the treatment and/or prevention of medical disorders where a decreased activity of hormone-sensitive lipase is desirable.
-
Porphyrin-Based Conjugated Microporous Polymer Tubes: Template-Free Synthesis and A Photocatalyst for Visible-Light-Driven Thiocyanation of Anilines作者:Pengfei Zhang、Yucheng Yin、Zhengxin Wang、Chunyang Yu、Yizhou Zhu、Deyue Yan、Weimin Liu、Yiyong MaiDOI:10.1021/acs.macromol.1c00190日期:2021.4.13photocurrent, compared to those of the irregular solid CMP counterpart. When serving as a metal-free photocatalyst for an undocumented visible-light-driven thiocyanation of anilines, CMP-1 exhibits excellent photocatalytic performance, with single chemoselectivity and high yields for the conversion of 25 types of anilines at ambient conditions. This study fills in the gap of the tubular morphological engineering
-
C-terminal modified oxamyl dipeptides as inhibitors of the ICE-ced-3 family of cysteine proteases申请人:——公开号:US20020042376A1公开(公告)日:2002-04-11This invention is directed to novel oxamyl dipeptide ICE/ced-3 family inhibitor compounds. The invention is also directed to pharmaceutical compositions containing these compounds, as well as to the use of such compositions in the treatment of patients suffering inflammatory, autoimmune and neurodegenerative diseases, for the prevention of ischemic injury, and for the preservation of organs that are to undergo a transplantation procedure.
表征谱图
-
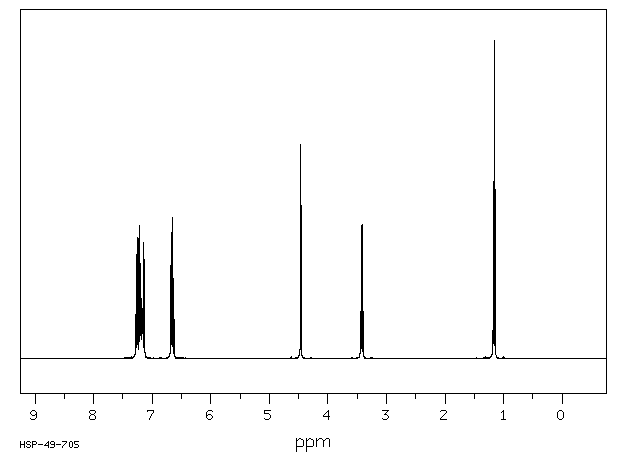
氢谱1HNMR
-
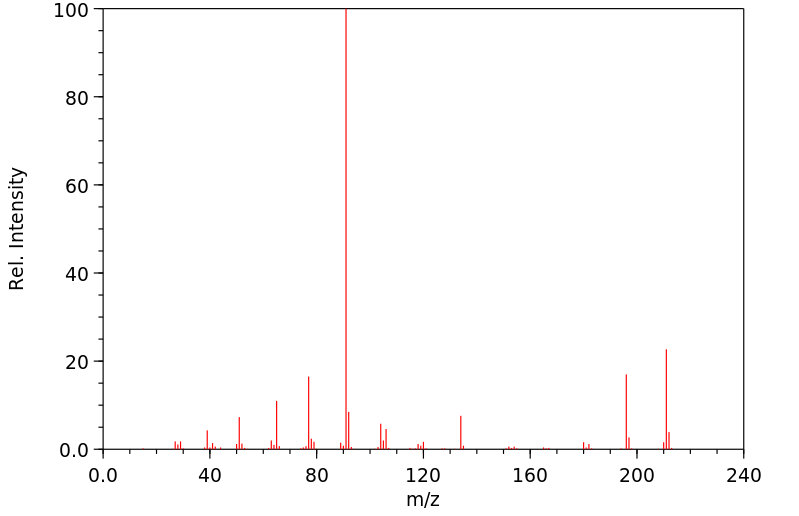
质谱MS
-
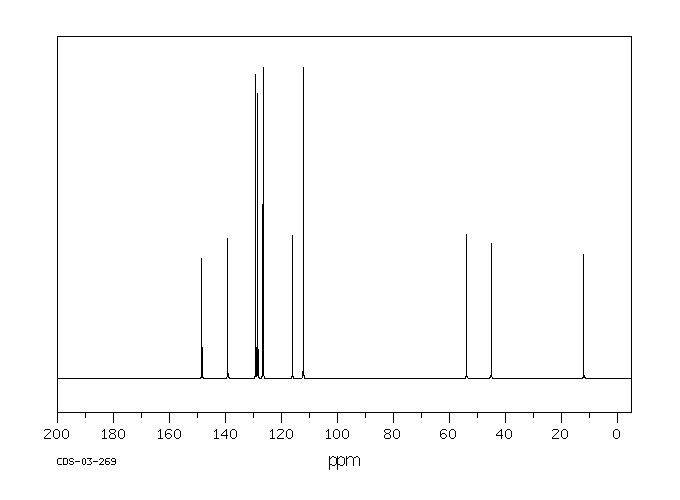
碳谱13CNMR
-
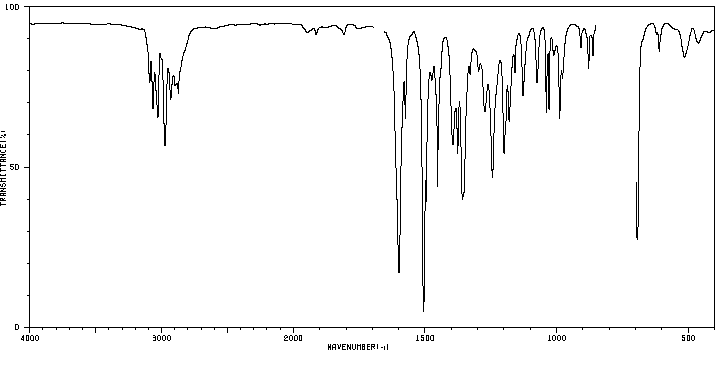
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息