双戊烯 | 138-86-3
中文名称
双戊烯
中文别名
1-甲基(1-甲基乙烯基)环己烯;二烯萜;(±)-柠檬烯;1,8-萜二烯;苎烯;柠蒙烯;苧烯;柠檬烯
英文名称
d-limonene
英文别名
limonene;Limonene, (+/-)-;1-methyl-4-prop-1-en-2-ylcyclohexene
CAS
138-86-3
化学式
C10H16
mdl
MFCD00062992
分子量
136.237
InChiKey
XMGQYMWWDOXHJM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-84--104 °C
-
沸点:170-180 °C (lit.)
-
密度:0.86 g/mL at 20 °C (lit.)
-
蒸气密度:4.7 (vs air)
-
闪点:119 °F
-
溶解度:氯仿:微溶
-
介电常数:2.3(20℃)
-
LogP:4.57
-
物理描述:Dipentene appears as a colorless liquid with an odor of lemon. Flash point 113°F. Density about 7.2 lb /gal and insoluble in water. Hence floats on water. Vapors heavier than air. Used as a solvent for rosin, waxes, rubber; as a dispersing agent for oils, resins, paints, lacquers, varnishes, and in floor waxes and furniture polishes.
-
颜色/状态:Colorless liquid
-
气味:Pleasant lemon-like
-
味道:Sweet, citrus taste
-
蒸汽密度:4.7 (Air = 1)
-
蒸汽压力:1.55 mm Hg at 25 °C /extrapolated/
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、强酸、卤代烃、卤素。
-
避免接触的条件:潮湿空气、受热。
-
聚合危害:可能发生聚合。
-
-
旋光度:Specific optical rotation: +94 deg to +99 deg at 25 °C/D
-
自燃温度:458 °F (237 °C)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
燃烧热:-19,520 Btu/lb = -10,840 cal/g = -454X10+5 J/kg
-
汽化热:140 Btu/lb = 77 cal/g = 3.2x10+5 J/kg
-
表面张力:26 dynes/cm = 0.026 N/m at 20 °C
-
折光率:Index of refraction: 1.4744 at 25 °C/D
-
保留指数:1020 ;1020 ;1022 ;1028 ;1023 ;1020 ;1022 ;1022 ;1005 ;1054 ;1026 ;1029 ;1021 ;1053 ;1020 ;1020 ;1024 ;1021 ;1025 ;1016 ;1022 ;1019 ;1064 ;1020 ;1020 ;1058 ;1039 ;1030 ;1020 ;1012 ;1025 ;1025 ;1026 ;1022 ;1022 ;1017 ;1032 ;1017 ;1019 ;1018 ;1021 ;1020 ;1020 ;1018 ;1029 ;1022 ;1020 ;1024 ;1020 ;1020 ;1012 ;1022 ;1020 ;1031 ;1021 ;1023 ;1023 ;1019 ;1010 ;1020 ;1022 ;1020 ;1025 ;1025 ;1013 ;1020 ;1020 ;1019 ;1033 ;1013 ;1015.8 ;1015.8 ;1020 ;1026 ;1026 ;1021 ;1019 ;1018 ;1023 ;1023 ;1021 ;1031 ;1023 ;1023 ;1025 ;1025 ;1025 ;1023 ;1030.8 ;1038 ;1030 ;1021 ;1021 ;1025 ;1016 ;1016 ;1022 ;1020 ;1020 ;1015 ;1028 ;1023 ;1021 ;1021 ;1023 ;1026 ;1024 ;1033.2 ;1021 ;1021 ;1020 ;1019 ;1026 ;1018 ;1021 ;1031 ;1026 ;1027 ;1025 ;1021 ;1030 ;1015 ;1031 ;1032 ;1026 ;1022 ;1023 ;1022 ;1018 ;1025 ;1023 ;1032 ;1023 ;1020 ;1023 ;1026 ;1019 ;1035 ;1029 ;1030 ;1020 ;1027 ;1022 ;1019.5 ;1021.5 ;1024.9 ;1026.3 ;1031.9 ;1026.3 ;1030.73 ;1026 ;1033 ;1034 ;1017 ;1021 ;1026 ;1029 ;1030 ;1031 ;1031 ;1034 ;1017 ;1018 ;1032 ;1032 ;1022 ;1011.1 ;1013.66 ;1016.32 ;1018.99 ;1021.74 ;1024.53 ;1027.41 ;1030.32 ;1033.31 ;1036.39 ;1039.52 ;1022 ;1022 ;1020 ;1023 ;1017 ;1017 ;1024 ;1041 ;1025 ;1033.04 ;1033.21 ;1039.19 ;1039.68 ;1024 ;1024 ;1025 ;1024 ;1039 ;1017 ;1019 ;1018 ;1031 ;1024 ;1019 ;1016 ;1015 ;1025 ;1024 ;1024 ;1024 ;1024 ;1021.75 ;1024.42 ;1027.23 ;1030.21 ;1033.38 ;1036.74 ;1040.32 ;1044.15 ;1023 ;1018 ;1020 ;1014 ;1028 ;1025 ;1021 ;1019 ;1024 ;1024 ;1026 ;1025 ;1024 ;1027 ;1026 ;1030 ;1043 ;1020 ;1020 ;1024 ;1025 ;1028 ;1026 ;1027 ;1027.1 ;1026 ;1029 ;1025 ;1019 ;1020 ;1025 ;1021 ;1026 ;1020 ;1027 ;1030 ;1022 ;1038 ;1020 ;1020 ;1030.5 ;1020 ;1020 ;1030 ;1030 ;1036 ;1040 ;1045 ;1049 ;1040 ;1019.8 ;1026 ;1029 ;1032 ;1036 ;1019 ;1036 ;1023 ;1038 ;1018 ;1018 ;1029 ;1030 ;1053 ;1027 ;1031.3 ;1035.2 ;1030 ;1031 ;1023 ;1024 ;1051 ;1042.8 ;1043 ;1028 ;1009 ;1025 ;1022 ;1022 ;1025 ;1025 ;1026 ;1026 ;1017 ;1033 ;1031 ;1032 ;1033 ;1032 ;1025 ;1020 ;1021 ;1025 ;1026 ;1054 ;1024 ;1021 ;1021 ;1024 ;1020 ;1018 ;1020 ;1027 ;1018 ;1035 ;1018 ;1025 ;1028 ;1031 ;1025 ;1023 ;1009 ;1009 ;1021 ;1023 ;1020 ;1020 ;1016 ;1015 ;1022 ;1020 ;1025 ;1027 ;1015 ;1009 ;1024 ;1029 ;1035 ;1022 ;1022 ;1030 ;1022 ;1026 ;1009 ;1016 ;1026 ;1009 ;1024 ;1032 ;1030 ;1018 ;1031 ;1026 ;1020 ;1019 ;1028 ;1029 ;1029 ;1014 ;1024 ;1009 ;1048 ;1025 ;1020 ;1020 ;1022 ;1015 ;1021 ;1023 ;1018 ;1018 ;1020 ;1021 ;1021 ;1021 ;1009 ;1019 ;1029 ;1024 ;1024 ;1020 ;1023 ;1032 ;1021 ;1030 ;1031 ;1028 ;1024 ;1029 ;1023 ;1031 ;1029 ;1038 ;1012 ;1019 ;1017 ;1020 ;1016 ;1009 ;1025 ;1034 ;1027 ;1024 ;1035 ;1029 ;1009 ;1020 ;1009 ;1020 ;1014 ;1017 ;1030 ;1024 ;1030 ;1026 ;1026 ;1025 ;1029 ;1029 ;1029 ;1013 ;1027 ;1025 ;1012 ;1016 ;1020 ;1017 ;1029 ;1023 ;1039 ;1026 ;1028 ;1014 ;1017 ;1017 ;1029 ;1025 ;1025 ;1025 ;1009 ;1030 ;1024 ;1026 ;1022 ;1015 ;1015 ;1017 ;1020 ;1020 ;1024 ;1024 ;1013 ;1033 ;1033 ;1023 ;1020 ;1021 ;1024 ;1039 ;1020 ;1025 ;1031 ;1009 ;1024 ;1021.1 ;1017 ;1019 ;1028 ;1015 ;1032 ;1020 ;1021 ;1033 ;1022 ;1019 ;1027 ;1006 ;1021 ;1021 ;1008 ;1040 ;1041 ;1027.1 ;1034.1 ;1017 ;1015 ;1016 ;1022 ;1022 ;1022 ;1022 ;1024 ;1020 ;1024 ;1024 ;1010 ;1030 ;1028 ;1024 ;1028 ;1030 ;1020 ;1014 ;1024 ;1022 ;1020 ;1012 ;1016 ;1025 ;1022 ;1019 ;1024 ;1009 ;1019 ;1023 ;1025 ;1024 ;1021 ;1019 ;1020 ;1020 ;1020 ;1020 ;1015 ;1026 ;1029 ;1030 ;1030 ;1021 ;1028 ;1016 ;1015 ;1029.1 ;1009 ;1030 ;1013 ;1014 ;1018 ;1027 ;1031 ;1023 ;1024 ;1009 ;1025 ;1026 ;1021 ;1026 ;1027 ;1022.1 ;1021 ;1020 ;1026 ;1024 ;1033.7 ;1012 ;1024 ;1025 ;1018 ;1020 ;1015 ;1022 ;1009 ;1026 ;1030 ;1024 ;1030.3 ;1022 ;1009 ;1020 ;1022 ;1025 ;1021 ;1028 ;1025 ;1025 ;1009 ;1026 ;1018 ;1018 ;1031 ;1025 ;1021 ;1022 ;1009 ;1021 ;1028 ;1021 ;1021 ;1027 ;1029 ;1017 ;1017 ;1017 ;1009 ;1009 ;1024 ;1023 ;1038 ;1039 ;1009 ;1025 ;1030 ;1029 ;1019 ;1009 ;1033 ;1022 ;1020 ;1022 ;1024 ;1025 ;1031 ;1019 ;1024 ;1021 ;1020 ;1021 ;1020 ;1020 ;1024 ;1025 ;1026 ;1026 ;1023 ;1020 ;1020 ;1027 ;1020 ;1019 ;1030 ;1030 ;1039 ;1025 ;1022 ;1024 ;1012 ;1029 ;1023 ;1030 ;1019 ;1024 ;1029 ;1019 ;1020 ;1020 ;1027 ;1028 ;1022 ;1029 ;1032 ;1014.4 ;1020.3 ;1020.3 ;1021.1 ;1021.4 ;1020 ;1021 ;1020 ;1020 ;1020 ;1019 ;1020 ;1029 ;1024 ;1027 ;1030 ;1022 ;1019 ;1014 ;1020 ;1020 ;1030 ;1020 ;1021 ;1032 ;1019 ;1019 ;1020 ;1019 ;1019 ;1019 ;1016 ;1019 ;1020 ;1013 ;1019 ;1018 ;1030 ;1031 ;1019 ;1053 ;1021 ;1022 ;1024 ;1008 ;1020 ;1002.4
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
口服给药后,大鼠和家兔尿液中的主要代谢物是过氧酸8,9-二醇,仓鼠中的是过氧基-β-D-吡喃葡萄糖苷酸,狗中的是p-薄荷-1-烯-8,9-二醇,豚鼠和人体中的是8-羟基-p-薄荷-1-烯-9-基-β-D-吡喃葡萄糖苷酸。
After oral administration, major metabolite in urine was perillic acid 8,9-diol in rats and rabbits, perillyl-beta-d-glucopyranosiduronic acid in hamsters, p-menth-1-ene-8,9-diol in dogs, and 8-hydroxy-p-menth-1-en-9-yl-beta-d-glucopyranosiduronic acid in guinea pigs and man.
来源:Hazardous Substances Data Bank (HSDB)
代谢
从兔尿中分离出的代谢物被鉴定为p-薄荷-1,8-二烯-10-醇。
... A metabolite isolated from rabbit urine was identified as p-mentha-1,8-dien-10-ol. ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
口服给予人类的柠檬烯会产生以下主要血浆代谢物:过氧酸、柠檬烯-1,2-二醇、柠檬烯-8,9-二醇和二氢过氧酸,这些可能来源于过氧酸。柠檬烯(未改变)和过氧酸伪影(甲基酯)也被检测为次要血浆代谢物。除柠檬烯-8,9-二醇在给药后一小时达到峰值外,所有代谢物的血浆峰值水平都在给药后4-6小时达到。在人类志愿者的尿液中已经鉴定出所有主要和次要代谢物的II期葡萄糖醛酸结合物。它们包括过氧酸、二氢过氧酸、柠檬烯-8,9-二醇、柠檬烯-10-醇、柠檬烯-6-醇和柠檬烯-7-醇(香叶醇)的葡萄糖醛酸结合物。
Limonene given orally to humans yields the following major plasma metabolites: perillic acid, limonene-1,2-diol, limonene-8,9-diol, and dihydroperillic acid, probably derived from perillic acid. Limonene (unchanged) and perillic acid artifacts (methyl ester) were also detected as minor plasma metabolites. Peak plasma levels for all metabolites were achieved 4-6 hours after administration, with the exception of limonene-8,9-diol which reached its peak level one hour after administration. Phase II glucuronic acid conjugates have been identified in the urine of human volunteers for all major and minor metabolites. They include the glucuronic acid conjugates of perillic acid, dihydroperillic acid, limonene-8,9-diol, limonene-10- ol, limonene-6-ol, and limonene-7-ol (perillyl alcohol).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:柠檬烯是一种无色液体。它目前在美国没有注册为农药使用,但批准的农药用途可能会定期更改,因此必须咨询联邦、州和地方当局以获取当前批准的用途。柠檬烯在工业涂装前用于去除金属油污的溶剂,用于电子和印刷行业的清洁,以及在油漆中作为溶剂。柠檬烯还用作食品、家用清洁产品和香水的风味和香气添加剂。它还用作胆结石溶剂。人类暴露和毒性:柠檬烯对人类皮肤有刺激性。柠檬烯的氧化形式已知会引起过敏性接触性皮炎。柠檬烯液体据报道会刺激眼睛,摄入会导致消化道刺激。如果摄入足够的量,可能会出现蛋白尿和血尿。它还与口腔和喉咙刺激、呼吸急促和肺功能受损有关。动物研究:柠檬烯对实验动物皮肤有刺激性。在口服或腹膜内给药后,动物的关键器官(雄性大鼠除外)是肝脏。暴露于柠檬烯会影响不同肝脏酶的数量和活性、肝脏重量、胆固醇水平和胆汁流量。这些变化在小鼠、大鼠和狗身上已有所观察。在体外实验中,使用不同菌株的沙门氏菌属伤寒杆菌进行的测试中,柠檬烯及其环氧化合物在浓度为0.3-3333微克/平板时未表现出诱变性,无论是否存在代谢激活。当与叙利亚仓鼠胚胎细胞一起孵化,浓度高达100微克/毫升或3毫摩尔时,柠檬烯未诱导统计学上显著的细胞转化。没有证据表明,在不存在母体毒性的情况下,柠檬烯具有致畸性或产生胚胎毒性效应。生态毒性研究:陆生生物最可能通过空气暴露于柠檬烯。使用蒸汽暴露对陆生物种(即昆虫)的少数研究在 ppm 级别发现了柠檬烯的影响。在水生环境中,柠檬烯对鱼类和大型溞表现出高急性毒性。
IDENTIFICATION AND USE: Limonene is a colorless liquid. It is not registered for current pesticide use in the U.S., but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses. Limonene is used as a solvent in degreasing metals prior to industrial painting, for cleaning in the electronic and printing industries, and in paint as a solvent. Limonene is also used as a flavor and fragrance additive in food, household cleaning products and perfumes. It is also used as gallstone solubilizer. HUMAN EXPOSURE AND TOXICITY: Limonene is a skin irritant in humans. The oxidized forms of limonene are known to cause allergic contact dermatitis. Limonene liquid has been reported to irritate eyes, ingestion causes irritation of GI tract. Albuminuria and hematuria are probable if ingested in sufficient quantity. It is also associated with mouth and throat irritation, shortness of breath, and impaired lung function. ANIMAL STUDIES: Limonene is a skin irritant in experimental animals. The critical organ in animals (except for male rats) following oral or ip administration is the liver. Exposure to limonene affects the amount and activity of different liver enzymes, liver weight, cholesterol levels and bile flow. These changes have been noted in mice, rats and dogs. Limonene and its epoxides were not mutagenic when tested at concentrations of 0.3-3333 ug/plate in in vitro assays using different strains of Salmonella typhimurium, in the presence or absence of metabolic activation. When incubated with Syrian hamster embryo cells up to 100 ug/mL or 3 mM, limonene did not induce statistically significant cell transformation. There is no evidence that limonene was teratogenic or produced embryotoxic effects in the absence of maternal toxicity. ECOTOXICITY STUDIES: Terrestrial organisms are most likely exposed to limonene via the air. The few studies of terrestrial species (i.e. insects) using vapor exposure revealed effects of limonene at ppm levels. In the aquatic environment, limonene exhibits high acute toxicity to fish and Daphnia.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:对于d-柠檬烯在人类中的致癌性,证据不足。在实验动物中,d-柠檬烯的致癌性有足够证据。总体评估:在对其在人类中的致癌性进行总体评估时,工作组认为d-柠檬烯通过一种与非DNA反应的alpha-2u-球蛋白相关的反应,在雄性大鼠中产生肾小管肿瘤。因此,d-柠檬烯增加雄性大鼠肾小管肿瘤发生率的机制与人类不相关。d-柠檬烯的致癌性在人类中无法分类(第3组)。/d-柠檬烯/
Evaluation: There is inadequate evidence in humans for the carcinogenicity of d-limonene. There is sufficient evidence in experimental animals for the carcinogenicity of d-limonene. Overall evaluation: In making its overall evaluation of the carcinogenicity to humans of d-limonene, the Working Group concluded that d-limonene produces renal tubular tumors in male rats by a non-DNA reactive alpha-2u-globulin associated response. Therefore, the mechanism by which d-limonene incr the incidence of renal tubular tumors in male rats is not relevant to humans. d-Limonene is not classifiable as to its carcinogenicity to humans (Group 3). /d-Limonene/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
神经毒素 - 急性溶剂综合征
Neurotoxin - Acute solvent syndrome
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
LC50(小鼠)= 67,500 毫克/立方米
LC50 (mice) = 67,500 mg/m3
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
吸入臭氧(O3),一种高度有毒的环境污染物,会导致气道炎症并加剧哮喘。然而,在室内空气中,O3与萜烯(环烯烃)反应,形成刺激气道的污染物。该研究的目的是探讨吸入O3与萜烯橙花油反应产物以及橙花油和低水平O3本身是否会在亚慢性小鼠吸入模型中诱导过敏性致敏(特异性免疫球蛋白[Ig]E的形成)和气道炎症,并且结合模型过敏原卵清蛋白(OVA)。BALB/cJ小鼠每周5天,连续2周,之后每周一次,共12周,仅通过吸入暴露。暴露于低剂量OVA结合O3、橙花油或橙花油/O3反应产物。OVA单独和OVA + Al(OH)3作为对照组。随后,所有组别连续三天暴露于高剂量OVA溶液。24小时后收集血清和支气管肺泡灌洗液。橙花油本身并未促进OVA特异性IgE或白细胞炎症。低水平O3促进了嗜酸性气道炎症,但未促进OVA特异性IgE的形成。橙花油/O3的反应产物促进了过敏性(OVA特异性IgE)致敏,但没有观察到过敏性哮喘的特征性肺炎症。总之,该研究不支持室内环境中O3引发的橙花油反应产物具有过敏性炎症效应。
Inhalation of ozone (O3), a highly toxic environmental pollutant, produces airway inflammation and exacerbates asthma. However, in indoor air, O3 reacts with terpenes (cyclic alkenes), leading to formation of airway irritating pollutants. The aim of the study was to examine whether inhalation of the reaction products of O3 and the terpene, limonene, as well as limonene and low-level O3 by themselves, induced allergic sensitization (formation of specific immunoglobulin [Ig] E) and airway inflammation in a subchronic mouse inhalation model in combination with the model allergen ovalbumin (OVA). BALB/cJ mice were exposed exclusively by inhalation for 5 d/wk for 2 wk and thereafter once weekly for 12 wk. Exposures were low-dose OVA in combination with O3, limonene, or limonene/O3 reaction products. OVA alone and OVA + Al(OH)3 served as control groups. Subsequently, all groups were exposed to a high-dose OVA solution on three consecutive days. Serum and bronchoalveolar lavage fluid were collected 24 hr later. Limonene by itself did not promote neither OVA-specific IgE nor leukocyte inflammation. Low-level O3 promoted eosinophilic airway inflammation, but not OVA-specific IgE formation. The reaction products of limonene/O3 promoted allergic (OVA-specific IgE) sensitization, but lung inflammation, which is a characteristic of allergic asthma, was not observed. In conclusion, the study does not support an allergic inflammatory effect attributed to O3-initiated limonene reaction products in the indoor environment.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
数据显示,单萜在胃肠道中的吸收不良。被吸收的碳氢化合物部分在亲脂性身体部位积累,然后被代谢并由肾脏排出。/单萜/
The data suggest that monoterpenes are poorly resorbed in the GI tract. The resorbed portion of hydrocarbons accumulates in the lipophilic body compartments and is metabolized and then excreted by the kidneys. /Monoterpenes/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
通过动物实验测量了泡沫浴中放射性柠檬烯的经皮吸收。在暴露10分钟后达到最大血药浓度,且浓度与暴露的皮肤面积成正比。
Percutaneous absorption of radioactive limonene from foam bath was measured in animals. Maximum blood level reached after 10 min of exposure and the concentration was proportional to the skin exposed.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
...SKH-1小鼠每天接受外用或口服橙油形式的柠檬烯,持续4周...在最后一次给药后4小时收集血浆和乳腺垫。小鼠处置研究显示,外用和口服橙油给药导致柠檬烯在乳腺组织中的分布相似,且没有临床毒性迹象...我们的研究表明,在小鼠外用橙油后,柠檬烯在乳腺组织中具有生物利用度...
...SKH-1 mice received topical or oral administration of limonene in the form of orange oil every day for 4 weeks ... Plasma and mammary pads were collected 4 hr after the final dosing. The mouse disposition study showed that topical and oral orange oil administration resulted in similar mammary tissue disposition of limonene with no clinical signs of toxicity ... Our studies showed that limonene is bio available in mammary tissue after topical orange oil application in mice ...
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:Xi,N
-
安全说明:S24,S37,S60,S61
-
危险类别码:R10,R50/53,R43,R38
-
WGK Germany:2
-
海关编码:2942000000
-
RTECS号:OS8350000
-
包装等级:III
-
危险类别:3
-
危险品运输编号:UN 2052 3/PG 3
-
储存条件:储存注意事项: - 储存在阴凉、通风良好的库房中。 - 远离火源和热源,库温不宜超过37℃。 - 包装需密封,避免与空气接触。 - 应与氧化剂和酸类分开存放,切忌混储。 - 不宜大量储存或长期存放。 - 使用防爆型照明和通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储存区应配备泄漏应急处理设备和合适的收容材料。
制备方法与用途
精油成分
柠檬烯(Limonene) 别名苎烯,也写作柠烯。是芸香科柑橘皮萃取的精油的主要成分之一,但并不是柑橘香气的主要来源。柠檬烯有三种同分异构体:天然的柠檬烯具有旋光性,分别是左旋柠檬烯和右旋柠檬烯;人工合成的柠檬烯不具旋光性,为消旋柠檬烯。在芳香有机化学中,左旋和右旋形式最为常见。
功效作用抗炎 柠檬烯能够抑制受损组织中炎症因子(如TNF、IL-6和IL-1β)的过度表达,同时提升IL-10(可抑制炎症反应的分子)活性,从而发挥抗炎效果。此外,它还能促进伤口愈合过程中胶原蛋白的合成,并减少炎症细胞的数量,帮助皮肤修复。
抗菌 柠檬烯能够提高受损组织中GSH-Px(谷胱甘肽过氧化物酶,一种重要的抗氧化酶,保护细胞免受氧化物损害)的活性,具有一定的抗氧化作用。
镇静 它通过增强5-羟色胺表达和减少多巴胺释放来抑制甲基苯丙胺诱导的运动神经活动,从而减轻神经元的过度兴奋,起到镇静安神的作用。
抗氧化 柠檬烯可以调节抗生素对产生耐药性的铜绿假单胞菌、大肠杆菌和金黄色葡萄球菌等细菌的抑菌效果,增强药效。例如,它能抑制某些耐药菌的EP(外排泵),帮助改善抗生素的耐药性,并增强其抗菌能力。
化学性质 用途- 用作合成橡胶、香料原料及溶剂。
- 常用其右旋体作为配制人造橙花、甜花、柠檬、香柠檬油的原料,并用于化妆、皂用及日用化学品香精中。在古龙型、花香型(如茉莉型)、薰衣草型以及松木、醛香、木香、果香或清香型配方中均适宜。
- 食品行业中作为修饰剂,用于白柠檬、果香及辛香等配方。
- 双戊烯可用作磁漆、假漆和各种含油树脂、树脂蜡、金属催干剂和溶剂;制造合成树脂和橡胶;调合橙花香精、柑桔油香精等;制成柠檬系精油的代用品。
- 柠檬烯定向氧化生成香芹酮;在无机酸存在下,与水加成反应生成α-松油醇和水合萜二醇;在铂或色催化剂作用下进行加氢反应生成对烷,并脱氢生成对伞花烃。此外,它还用作油类分散剂、橡胶添加剂及润湿剂等。
- 作为溶剂使用,也可用于香料合成和农药生产。
在制造本品时,可由上述精油进行分馏提取。也可通过松节油水合制备松油醇的过程中副产双戊烯。
分类易燃液体
毒性分级低毒
- 急性毒性:口服-大鼠 LD50: 5000 毫克/公斤
- 刺激数据:皮肤-兔子 500 毫克/24小时 中度
与空气混合可爆
可燃性危险特性遇明火、高温、氧化剂较易燃;燃烧产生刺激烟雾。
储运特性库房通风低温干燥;与氧化剂分开存放;不宜久储,以防聚合
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甜橙提取物 D-limonene 5989-27-5 C10H16 136.237 (S)-(-)-柠檬烯 (-)-(S)-limonene 5989-54-8 C10H16 136.237 紫苏醇 perillol 536-59-4 C10H16O 152.236 —— p-menth-1-ene 27966-26-3 C10H18 138.253 —— (-)-perillyl formate 29621-55-4 C11H16O2 180.247 —— (R)-Carvone 2244-16-8 C10H14O 150.221 香芹酮 Carvone 99-49-0 C10H14O 150.221 右旋香芹酮 (S)-p-mentha-6,8-dien-2-one 2244-16-8 C10H14O 150.221 三甲基((4-(丙-1-烯-2-基)环己-1-烯-1-基)甲基)硅烷 1-Trimethylsilylmethyl-4-isopropenylcyclohexene 82096-14-8 C13H24Si 208.419 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甜橙提取物 D-limonene 5989-27-5 C10H16 136.237 (S)-(-)-柠檬烯 (-)-(S)-limonene 5989-54-8 C10H16 136.237 —— (+/-)-lanceol 18681-09-9 C15H24O 220.355 4-甲基-γ-亚甲基3-环己烯-1-丙醇 3-(4-methylcyclohex-3-en-1-yl)but-3-en-1-ol 15766-66-2 C11H18O 166.263 柠檬烯-10-醇 Limonen-10-ol 3269-90-7 C10H16O 152.236 —— 2-(1'-methylcyclohexen-4'-yl)-2-propenyl bromide 60699-34-5 C10H15Br 215.133 紫苏醇 perillol 536-59-4 C10H16O 152.236 l-紫苏醛 perillaldehyde 2111-75-3 C10H14O 150.221 —— Perillyl chloride 127940-66-3 C10H15Cl 170.682 —— vestitenone 69401-36-1 C12H18O 178.274 —— 10-acetyllimonene 64615-30-1 C12H18O 178.274 —— 4-(4-Methyl-3-cyclohexen-1-yl)-4-pentenoic acid 38970-45-5 C12H18O2 194.274 —— trans α-atlantone 532-64-9 C15H22O 218.339 —— p-menth-1-ene 27966-26-3 C10H18 138.253 (+)-对-薄荷-1-烯 (+)-p-menth-1-ene 1195-31-9 C10H18 138.253 —— 4-methyl-γ-methylene-α-(2-methylpropyl)-3-cyclohexene-1-propanol 37794-77-7 C15H26O 222.371 —— trans-α-Dihydro-12,13-atlanton 57130-01-5 C15H24O 220.355 —— 5-isopropenyl-2-methylcyclohex-2-enylmethanol 5962-49-2 C11H18O 166.263 —— (E/Z)-p-mentha-1,2(9)-diene-9-carboxylic acid 108448-75-5 C11H16O2 180.247 —— 4-methyl-β-methylene-3-cyclohexene-1-propanethioic acid 113435-69-1 C11H16OS 196.313 3-(4-甲基环己-3-烯基)丁-3-烯基乙酸酯 Limonenylcarbinolacetat 6819-19-8 C13H20O2 208.301 香芹醇 5-Isopropenyl-2-methyl-cyclohex-2-enol 99-48-9 C10H16O 152.236 —— (-)-trans-carveol 18383-51-2 C10H16O 152.236 —— S-(-)-carveol —— C10H16O 152.236 对薄荷-3-烯 3-p-menthene 500-00-5 C10H18 138.253 香芹酮 Carvone 99-49-0 C10H14O 150.221 —— 6-chloro-p-mentha-1,8-diene 71305-95-8 C10H15Cl 170.682 甲基环己烯基丁醇 3-(4-methyl-3-cyclohexen-1-yl)-1-butanol 15760-18-6 C11H20O 168.279 异辣薄荷烯酮 isopiperitenone 529-01-1 C10H14O 150.221 新柠檬醛; 3-(4-甲基-3-环己烯基)丁醛; beta,4-二甲基-3-环己烯-1-丙醛 1-Methyl-4-(2-butanalyl)-1-cyclohexene 6784-13-0 C11H18O 166.263 —— (4RS,8RS)-1-Methyl-4-(3-butanalyl)-1-cyclohexene —— C11H18O 166.263 —— (3S)-3-[(1S)-4-Methylcyclohex-3-enyl]butanal 138117-27-8 C11H18O 166.263 4-乙烯基-1-环己烯 4-Vinylcyclohexene 100-40-3 C8H12 108.183 1-亚甲基-4-(1-甲基乙烯基)环己烷 pseudolimonene 499-97-8 C10H16 136.237 —— (E)-ethyl 3-(4-methylcyclohex-3-en-1-yl)-2-butenoate 73416-67-8 C13H20O2 208.301 2-甲基-4-(4-甲基-3-环己烯-1-基)戊醛 2-methyl-4-(4-methylcyclohex-3-ene-1-yl)pentanal 79347-73-2 C13H22O 194.317 (+)-p-薄荷-1-烯-9-醇 menth-1-en-9-ol 18479-68-0 C10H18O 154.252 2-(4-甲基-3-环己烯-1-基)丙醛 α-4-dimethyl-3-cyclohexene-1-acetaldehyde 29548-14-9 C10H16O 152.236 —— 1-methyl-4-(1-methylcyclopropyl)-1-cyclohexene 60059-22-5 C11H18 150.264 —— 2-methyl-5-[(E)-6-methyl-4-oxohept-2-en-2-yl]cyclohex-2-en-1-one 57095-92-8 C15H22O2 234.338 4-(1-甲基乙烯基)-1-环己烯-1-甲醇乙酸酯 perillyl acetate 15111-96-3 C12H18O2 194.274 —— 1,1,1-trichloro-4-(4-methylcyclohex-3-enyl)pent-4-en-2-ol 90454-95-8 C12H17Cl3O 283.625 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:MORIKAVA, TOSIYUKI;TAKAXASI, KEHJ摘要:DOI:
-
作为产物:描述:(R)-氧化柠檬烯 以86%的产率得到参考文献:名称:GURUDUTT K. N.; RAVINDRANATH B., TETRAHEDRON LETT., 1980, 21, NO 12, 1173-1174摘要:DOI:
-
作为试剂:描述:四氟乙烯 、 一氟三氯甲烷 在 aluminum chlorofluoride 双戊烯 作用下, 15.4~40.2 ℃ 、144.77 kPa 条件下, 反应 0.72h, 生成 四氯化碳 、 1,1,1,3-四氯四氟丙烷 、 二氯二氟甲烷 、 1-氯-1,1,2,2,3,3,3-七氟丙烷 、 1,1,1-三氯五氟丙烷 、 1,1,3-三氯五氟丙烷 、 3,3-dichloro-1,1,1,2,2,4,4,5,5,5-decafluoropentane 、 CClF2CF2CCl2CF2CF3参考文献:名称:WO2008/54780摘要:公开号:
文献信息
-
The kinetics, stereochemistry, and deuterium isotope effects in the α-pinene pyrolysis. Evidence for incursion of multiple conformations of a diradical作者:Joseph J Gajewski、Ilya Kuchuk、Christopher Hawkins、Robert StineDOI:10.1016/s0040-4020(02)00676-2日期:2002.8α-pinene at 256.7°C for 2400 s gave dipentene with twice as much deuterium as hydrogen transfer with kH/kD=1.49 and alloocimine with a Z and E trideuteriomethyl ratio of ca. 5 with kH/kD=0.89. The isotope effect on loss of starting material was 1.16. Separation of the enantiomers of α-pinene from 3600 s pyrolyses at 256.7°C followed by NMR analysis revealed that the ratio of the R-syn to R-anti to S-anti旋光性α-pine烯的热解得到95%的外消旋柠檬烯(二戊烯),别甲胺,外消旋的α-pine烯,α-py烯。报告激活参数。(S)syn -6-trideuteriomethylα-pinene在256.7°C的温度下热解2400 s,生成的二戊烯的氘量是氢转移的两倍,k H / k D = 1.49,Z和E的三异氘甲基比值约26。5 ķ ħ / ķ d = 0.89。同位素对原料损失的影响为1.16。在256.7°C下从3600 s热解中分离出α-pine烯的对映体,然后进行NMR分析,结果表明[R -顺式到- [R -抗到小号-反异构体是4.6:3.7:1,在大约两个半衰期。动力学分析表明,先前提出的涉及两个具有C s对称性的双自由基缓慢相互转化的转化机理与α-pine烯异构体的分布不一致,特别是与键相比更多的表面保留产物(R - anti)的形成-旋转异构体(S - anti)。另一个的
-
Substituted bicyclooctenemethanols申请人:International Flavors & Fragrances Inc.公开号:US04166916A1公开(公告)日:1979-09-04The use of unsaturated bicyclooctenemethanols to augment or enhance the organoleptic properties of perfumes, tobaccos and perfumed articles and particularly the aroma of perfumes and perfumed articles and the aroma and taste of tobaccos, together with compositions containing such bicyclooctenemethanols and processes for preparing them, said bicyclooctenemethanols having the generic structures: ##STR1## wherein the wavy line represents exo or endo configurations of the ethanol moiety with respect to the carbon-carbon double bond of the bicyclooctene moiety; and intermediates useful in processes for preparing such bicyclooctenemethanols which intermediates have the structures: ##STR2## wherein X is a halogen selected from the group consisting of chlorine, bromine and iodine.
-
Substituted bicyclooctenemethanols, process for producing same and uses申请人:International Flavors & Fragrances Inc.公开号:US04163737A1公开(公告)日:1979-08-07The use of unsaturated bicyclooctenemethanols to augment or enhance the organoleptic properties of perfumes, particularly the aroma of perfumes together with compositions containing such bicyclooctenemethanols and processes for preparing them, said bicyclooctenemethanols having the generic structures: ##STR1## wherein the wavy line represents exo or endo configurations of the ethanol moiety.
-
Sweetening with cycloalkyl ethers and thioethers of dipeptides申请人:General Foods Corporation公开号:US04676989A1公开(公告)日:1987-06-30The following dipeptides possess a high order of sweetness: ##STR1## wherein X=O or S; R is alkyl containing 1-3 carbon atoms; R.sub.1 is cycloalkyl, cycloalkenyl, lower alkyl-substituted cycloalkyl or cycloalkenyl, bicycloalkyl, bicycloalkenyl, tricycloalkyl, cyclic ether, cyclic thioether, cyclic sulfoxides, cyclic sulfones, aryl, benzyl, alkylaryl, aromatic heterocyclic or alkyl substituted aromatic heterocyclic containing up to 10 ring carbon atoms and up to a total of 12 carbon atoms; R.sub.2, R.sub.3, R.sub.4 and R.sub.6 are each H or lower alkyl; R.sub.5 is H, lower alkyl or cycloalkyl containing 3-5 ring carbons; each n=0, 1 or 2; m=0 or 1; Z is an alkylene chain containing 0-2 carbon atoms in the principal chain and up to a total of 6 carbon atoms; and food-acceptable salts.
-
Process for augmenting or enhancing the aroma of a detergent using申请人:International Flavors & Fragrances Inc.公开号:US04267067A1公开(公告)日:1981-05-12Described is a process for augmenting or enhancing the aroma of a solid or liquid anionic, cationic or nonionic detergent comprising the step of intimately admixing with a solid or liquid anionic, cationic or nonionic detergent base an aroma augmenting or enhancing quantity of 1,3,5,5-tetramethyl-2-oxabicyclo[2.2.2]octane.本发明涉及一种增强固体或液体阴离子、阳离子或非离子洗涤剂的香气的方法,其中包括将1,3,5,5-四甲基-2-氧杂双环[2.2.2]辛烷的增香或增强量与固体或液体阴离子、阳离子或非离子洗涤剂基料混合。
表征谱图
-
氢谱1HNMR
-
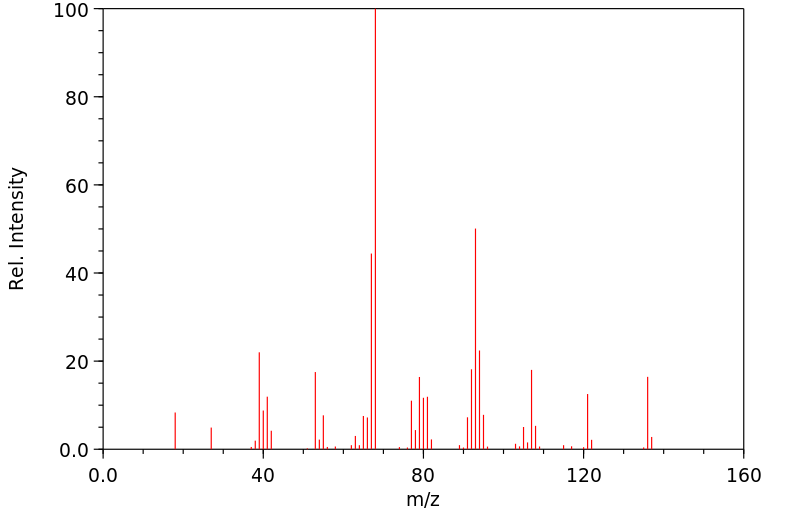
质谱MS
-
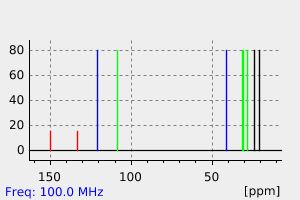
碳谱13CNMR
-
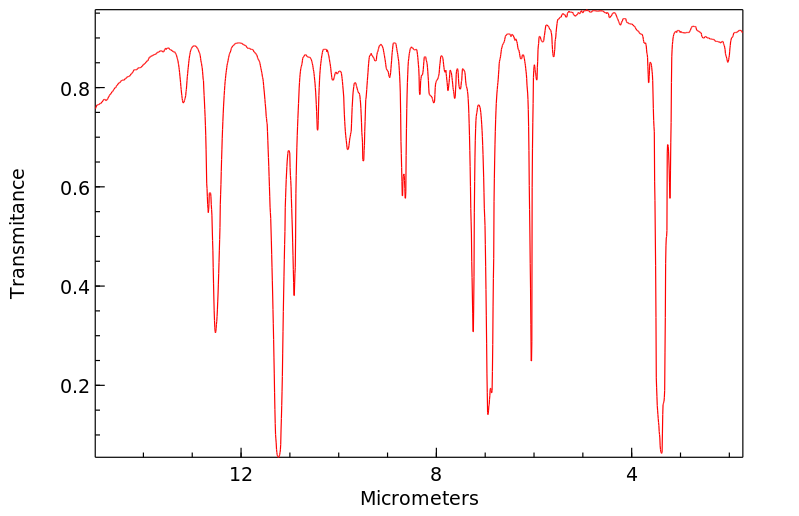
红外IR
-
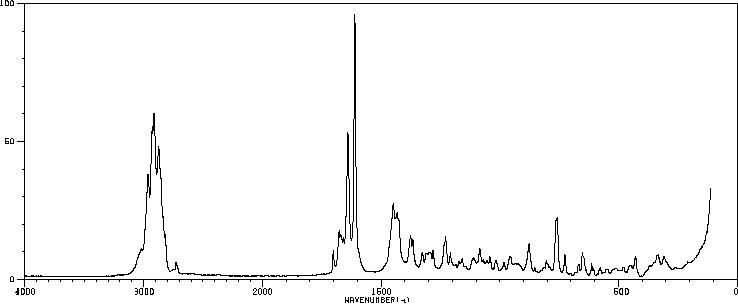
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸