反式-查耳酮 | 614-47-1
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:55-59 °C
-
沸点:208 °C25 mm Hg(lit.)
-
密度:1.0712
-
闪点:>230 °F
-
溶解度:可溶于氯仿(少许)
-
LogP:4.013 (est)
-
物理描述:Solid
-
保留指数:1970.6
-
稳定性/保质期:
在常温常压下,不会发生分解反应。
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S22,S36/37/39,S45
-
危险类别码:R22,R36/37
-
WGK Germany:3
-
海关编码:29143990
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:UD5576750
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:本品应密封存放在阴凉干燥处。请将密闭容器储存在主包装中,并置于阴凉、干爽的地方。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : trans-Chalcone
CAS-No. : 614-47-1
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Oral (Category 4)
Eye irritation (Category 2)
Specific target organ toxicity - single exposure (Category 3)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Irritating to eyes and respiratory system. Harmful if swallowed.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
Harmful if swallowed.
Causes serious eye irritation.
May cause respiratory irritation.
Precautionary statement(s)
Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R36/37 Irritating to eyes and respiratory system.
R22 Harmful if swallowed.
S-phrase(s)
S22 Do not breathe dust.
S36/37/39 Wear suitable protective clothing, gloves and eye/face protection.
S45 In case of accident or if you feel unwell, seek medical advice immediately
(show the label where possible).
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : Benzylideneacetophenone
Formula : C15H12O
Molecular Weight : 208,26 g/mol
Component Concentration
(E)-Chalcone
CAS-No. 614-47-1 -
EC-No. 210-383-8
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 55 - 57 °C - lit.
point
f) Initial boiling point and 208 °C at 33 hPa - lit.
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
LD50 Oral - mouse - 1.048 mg/kg
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Causes respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes Causes serious eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反式-查耳酮是一种不饱和酮类化合物,呈现为浅黄色斜方棱柱体结晶。据文献报道,它是一种小分子GPR52拮抗剂,并可通过苯乙酮和苯甲醛一步反应制备得到。
制备在氮气保护下,将[Ni(dmpymt)₂] 6(5 mol%Ni)、KOH(1.0 mmol)、苯甲醇(1.5 mmol)与1-苯基乙醇(1.0 mmol)加入到装有2.5 mL甲苯和0.5 mL叔丁醇的50毫升Schlenk试管中。将试管置于70°C油浴中,以缓慢稳定的氮气流搅拌36小时。冷却至室温后,加入水(10毫升)。用CH₂Cl₂(3×10 mL)萃取水溶液,用无水Na₂SO₄干燥合并的萃取液,除去溶剂,在短时间快速柱色谱上纯化粗产物得到反式-查耳酮。
生物活性trans-Chalcone 是从Aronia melanocarpa 果皮中分离出来的,是类黄酮前体的双酚核心结构。它表现出有效的脂肪酸合酶 (FAS) 和 α-淀粉酶 (b-α-amylase) 抑制作用,并能引起乳腺癌细胞系 MCF-7 的凋亡,具有抗真菌和抗癌活性。
体外研究trans-Chalcone 对猪胰淀粉酶表现出竞争性抑制作用(Ki = 48 μM)。在24小时内,30.23至98.03 μM的浓度能诱导 MCF-7 细胞周期停滞和凋亡。此外,在24小时后,trans-Chalcone 还减少了 Bcl-2 的表达并诱导了 CIDEA 基因的表达,并在6小时内对Bcl-2表现出更强的抑制作用、诱导了 APAF1 和 BAX,并显著增强了 CIDEA 的表达。在24小时内,trans-Chalcone 抑制 MCF-7 细胞存活(IC₂₀ = 30.23 μM;IC₅₀ = 58.25 μM;IC₈₀ = 98.03 μM)。对于 MCF-7 和 3T3 细胞系,trans-Chalcone 的 IC₅₀ 分别为41.53 μM和48.41 μM。研究显示 trans-Chalcone 表现出显著的细胞毒性活性。
凋亡分析 细胞周期分析-
MCF-7 细胞
- 浓度:20, 40, 80 μM
- 孵育时间:24小时
- 结果:导致 G₁期细胞周期停滞。
-
MCF-7 细胞
- 浓度:58.25 μM
- 孵育时间:6, 24小时
- 结果:对 Bcl-2 的抑制作用更强、诱导了 APAF1 和 BAX,并强烈诱导了 CIDEA 在24小时内表达。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯亚甲基苯乙酮 benzalacetophenone 94-41-7 C15H12O 208.26 (2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)- (Z)-chalcone 614-46-0 C15H12O 208.26 (E)-3-(4-羟基苯基)-1-苯基丙-2-烯-1-酮 (E)-3-(4-hydroxyphenyl)-1-phenyl-2-propen-1-one 38239-55-3 C15H12O2 224.259 —— (E)-1-(4-hydroxyphenyl)-3-phenylprop-2-en-1-one 2657-25-2 C15H12O2 224.259 (2E)-1-(4-氯苯基)-3-苯基-2-丙烯-1-酮 (E)-1-(4-chlorophenyl)-3-phenyl-2-propen-1-one 22966-22-9 C15H11ClO 242.705 —— 3-hydroxychalcone 81226-95-1 C15H12O2 224.259 —— (2E)-1-phenyl-3-[4-(trifluoromethyl)phenyl]prop-2-en-1-one —— C16H11F3O 276.258 —— (Z)-3-amino-1,3-diphenylprop-2-en-1-one 98815-39-5 C15H13NO 223.274 —— 2-bromo-1,3-diphenyl-2-propen-1-one 6935-75-7 C15H11BrO 287.156 1-(2-氯苯基)-3-苯基丙-2-烯-1-酮 (E)-1-(2-chlorophenyl)-3-phenylprop-2-en-1-one 144017-77-6 C15H11ClO 242.705 —— (E)-1-(2-bromophenyl)-3-phenylprop-2-en-1-one 300657-41-4 C15H11BrO 287.156 1-苯基-2-丙烯基-1-酮 PVK 768-03-6 C9H8O 132.162 —— (1E)-1,3-diphenylpropene 3412-44-0 C15H14 194.276 3-硝基查耳酮 3-nitrochalcone 614-48-2 C15H11NO3 253.257 亚苄基二苯甲酰甲烷 2-benzylidene-1,3-diphenyl-propane-1,3-dione 5398-64-1 C22H16O2 312.368 —— (Z)-3-ethylsulfanyl-1,3-diphenylprop-2-en-1-one 78080-38-3 C17H16OS 268.379 —— 1-phenyl-3-dimethylaminoprop-2-enone 1201-93-0 C11H13NO 175.23 3-苯甲酰丙烯酸 3-benzoylacrylic acid 583-06-2 C10H8O3 176.172 —— α-Carboxychalcon 64235-44-5 C16H12O3 252.269 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)- (Z)-chalcone 614-46-0 C15H12O 208.26 苯亚甲基苯乙酮 benzalacetophenone 94-41-7 C15H12O 208.26 —— 3-(2-chlorophenyl)-1-phenylpropenone 3300-67-2 C15H11ClO 242.705 —— (Z)-3-amino-1,3-diphenylprop-2-en-1-one 98815-39-5 C15H13NO 223.274 —— dibenzoylmethane 198836-73-6 C15H12O2 224.259 —— (Z)-3-hydroxy-1,3-diphenyl-2-propen-1-one 62961-62-0 C15H12O2 224.259 —— (E)-2-deuterio-1,3-diphenyl-2-propen-1-one 14132-50-4 C15H12O 209.252 —— (E)-2-bromo-1,3-diphenylprop-2-en-1-one 32147-19-6 C15H11BrO 287.156 —— (Z)-2-bromo-1,3-diphenylprop-2-en-1-one 32147-20-9 C15H11BrO 287.156 —— (Z)-2-chloro-1,3-diphenylprop-2-en-1-one —— C15H11ClO 242.705 —— (Z)-1,3-diphenyl-2-fluoro-2-propen-1-one 41343-12-8 C15H11FO 226.25 —— 2-aminochalcone 78396-00-6 C15H13NO 223.274 —— (E)-3-(4-nitrophenyl)-1-phenylprop-2-en-1-one 2960-55-6 C15H11NO3 253.257 beta-苯基-查耳酮 1,3,3-triphenylprop-2-en-1-one 849-01-4 C21H16O 284.357 —— 1,3-diphenylpropene —— C15H14 194.276 —— (1E)-1,3-diphenylpropene 3412-44-0 C15H14 194.276 —— 1,3-diphenyl-3-(p-tolyl)prop-2-en-1-one 108511-11-1 C22H18O 298.384 —— 1c.3-diphenyl-1t-p-tolyl-propen-(1)-one-(3) 99142-94-6 C22H18O 298.384 —— Chalcon-4-sulfonsaeure —— C15H12O4S 288.324 —— (E)-1-(2-chlorophenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one —— C15H11ClO2 258.704 —— (Z)-3-(methylamino)-1,3-diphenylprop-2-en-1-one 28640-26-8 C16H15NO 237.301 —— 2-benzyl-1,3-diphenylprop-2-en-1-one 627875-72-3 C22H18O 298.384 —— 1,3,5-triphenyl-penta-2,4-dien-1-one 52762-76-2 C23H18O 310.395 —— (E,E)-1,3,5-triphenyl-penta-2,4-dien-1-one 52762-77-3 C23H18O 310.395 —— 3-(4-methoxyphenyl)-1,3-diphenylprop-2-en-1-one 108511-16-6 C22H18O2 314.384 —— (Z)-2-azido-1,3-diphenylprop-2-en-1-one 26087-01-4 C15H11N3O 249.272 —— 3-(4-methoxyphenyl)-1,3-diphenylprop-2-en-1-one 99142-95-7 C22H18O2 314.384 —— α-azidochalcone 26087-01-4 C15H11N3O 249.272 —— 3-(4-(dimethylamino)phenyl)-1,3-diphenylprop-2-en-1-one 1428541-51-8 C23H21NO 327.426 —— (Z)-3-(benzylamino)-1,3-diphenylprop-2-en-1-one 187606-82-2 C22H19NO 313.399 —— (E)-buta-1,3-diene-1,3-diyldibenzene 35632-82-7 C16H14 206.287 —— (Z)-1,3-diphenyl-3-(prop-2-yn-1-ylamino)prop-2-en-1-one —— C18H15NO 261.323 —— (E)-3-(2-nitrophenyl)-1-phenylprop-2-en-1-one 53744-31-3 C15H11NO3 253.257 —— (E)-1,3-diphenylprop-2-en-1-amine 85739-50-0 C15H15N 209.291 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:A novel two-step preparation of enaminoketones by amination of α,β-unsaturated ketones with methoxyamine摘要:beta-Methoxyaminoketones, derived from the addition of methoxyamine to 1,3-diaryl-2-propen-1-one, underwent base-induced beta-elimination to furnish the corresponding enaminoketones in good to moderate yields. The reaction conditions and substituents on the substrates significantly influenced the selectivity in the production of enaminoketone and/or aziridineketone. (C) 1998 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(98)01801-2
-
作为产物:描述:ω-苄基苯乙酮 在 二(氰基苯)二氯化钯 、 copper diacetate 、 N,N-二异丙基乙胺 作用下, 以 乙醚 、 正己烷 、 N,N-二甲基乙酰胺 为溶剂, 反应 4.0h, 生成 反式-查耳酮参考文献:名称:钯(II)催化生成硼烯醇盐的脱硼硼化摘要:Pd II催化了从酮和9-碘-9-硼环[3.3.1]壬烷生成的硼烯酸酯的硼氢化脱氢反应,提供了从酮到α,β-不饱和酮的综合用途协议。该反应中使用的Pd II化合物在Cu(OAc)2的存在下催化工作。观察到烯烃部分的高反选择性。尽管存在Pd物种,但卤化芳基部分(-Br和-Cl)对于该反应仍然完好无损。当使用化学计量的Pd II时,也可以使用酯底物。使用硼和甲硅烷基烯醇盐的交叉反应表明,氧化反应比Saegusa-Ito反应快得多。DOI:10.1002/chem.201604306
-
作为试剂:描述:三氟化硼乙醚 、 1,5-二苯基-3-(吡啶-4-基)戊烷-1,5-二酮 在 溶剂黄146 、 反式-查耳酮 作用下, 反应 6.0h, 以71%的产率得到2,6-diphenyl-4-<(1H)-pyridinium-4-yl>pyrylium bistetrafluoroborate参考文献:名称:ELECTROCHROMIC TWO-CORE VIOLOGEN DERIVATIVES AND OPTICAL ARTICLES CONTAINING THEM摘要:本发明涉及一组新型电致变色材料。更具体地,涉及具有双核紫精的电致变色材料,以及将这些双核紫精用作可变透射率介质制造光学制品,如眼镜镜片。公开号:US20160221949A1
文献信息
-
Additives and products including oligoesters申请人:——公开号:US20030199593A1公开(公告)日:2003-10-23The present invention relates to oligoesters and their use or the creation of additives. Oligoester containing additives and/or oligoesters themselves may be used for formulating pharmaceutical preparations, cosmetics or personal care products such as shampoos and conditioners. These oligoesters are particularly useful for the creation of multi-purpose additives that can impart conditioning, long substantivity and/or UV protection. Individual oligoesters and oligoester mixtures are described.本发明涉及寡酯及其用途或添加剂的制备。含有寡酯的添加剂和/或寡酯本身可用于配制药物制剂、化妆品或个人护理产品,如洗发水和护发素。这些寡酯对于制备能够赋予调理、长效性和/或紫外线保护的多功能添加剂特别有用。描述了单独的寡酯和寡酯混合物。
-
[EN] COMPOUNDS AND COMPOSITIONS COMPRISING CDK INHIBITORS AND METHODS FOR THE TREATMENT OF CANCER<br/>[FR] COMPOSÉS ET COMPOSITIONS COMPRENANT DES INHIBITEURS DES CDK ET MÉTHODES DE TRAITEMENT DU CANCER申请人:UNIV GEORGIA STATE RES FOUND公开号:WO2010129858A1公开(公告)日:2010-11-11Disclosed herein are compounds suitable for use as antitumor agents, methods for treating cancer wherein the disclosed compounds are used in making a medicament for the treatment of cancer, methods for treating a tumor comprising, administering to a subject a composition comprising one or more of the disclosed cytotoxic agents, and methods for preparing the disclosed antitumor agents.
-
[EN] SYNTHETIC ANALOGUES OF XANTHOHUMOL<br/>[FR] ANALOGUES SYNTHÉTIQUES DU XANTHOHUMOL申请人:UNIV PISA公开号:WO2014167481A1公开(公告)日:2014-10-16The present invention relates to novel synthetic analogues of xanthohumol and the use thereof.本发明涉及新型黄葛素合成类似物及其用途。
-
Synthesis of dihydroquinolinones <i>via</i> iridium-catalyzed cascade C–H amidation and intramolecular aza-Michael addition作者:Changduo Pan、Zhenkun Yang、Hao Xiong、Jiangang Teng、Yun Wang、Jin-Tao YuDOI:10.1039/c8cc09751h日期:——An iridium-catalyzed annulation of chalcones with sulfonyl azides via cascade C–H amidation and intramolecular aza-Michael addition was developed, affording a variety of 2-aryl-2,3-dihydro-4-quinolones in moderate to good yields. This reaction features easy operation, readily available starting materials, and the cascade formation of two C–N bonds in one pot.
-
ACYL-HYDRAZONE AND OXADIAZOLE COMPOUNDS, PHARMACEUTICAL COMPOSITIONS CONTAINING THE SAME AND USES THEREOF申请人:Universidade Federal de Santa Catarina公开号:US20150191445A1公开(公告)日:2015-07-09The present invention relates to acyl-hydrazone compounds, in particular 3,4,5-trimethoxyphenyl-hydrazide derivatives, as well as the oxadiazole analogs thereof and other similar compounds, and to the pharmaceutical use of the same for the treatment of various diseases associated with cell proliferation, such as leukemias, including acute lymphoblastic leukemia (ALL), tumours and inflammation. Acyl-hydrazones have been obtained having activity similar to that of the compound used as a standard in experiments (colchicine). The greater selectivity of the compounds according to the invention is an important feature, associated with fewer side effects than the pharmaceuticals used at present in clinical treatments. The synthetised acyl-hydrazones, more particularly the compounds 02 and 07, exhibited important antileukemic activity, which suggests 02 and 07 as candidates to pharmaceutical prototypes, or to pharmaceuticals for the treatment of leukemias, in particular acute lymphoblastic leukemia (ALL), tumours and other proliferative diseases, such as inflammation. The action mechanism of the most active compounds was determined by using DNA microarrays and subsequent tests indicated by the chip, besides selectivity studies in healthy human lymphocytes.本发明涉及酰基腙化合物,特别是3,4,5-三甲氧基苯基腙衍生物,以及其噁二唑类似物和其他类似化合物,以及它们在治疗与细胞增殖相关的各种疾病,如白血病(包括急性淋巴细胞白血病(ALL))、肿瘤和炎症方面的药用。已获得具有与实验中使用的化合物(秋水仙碱)相似活性的酰基腙。根据本发明的化合物具有更大的选择性,与目前在临床治疗中使用的药物相比,副作用更少是一个重要特征。合成的酰基腙,尤其是化合物02和07,表现出重要的抗白血病活性,这表明02和07可能成为药物原型的候选,或用于治疗白血病,特别是急性淋巴细胞白血病(ALL)、肿瘤和其他增殖性疾病,如炎症的药物。最活性化合物的作用机制是通过使用DNA微阵列确定的,并且通过芯片指示的后续测试,以及对健康人类淋巴细胞的选择性研究。
表征谱图
-
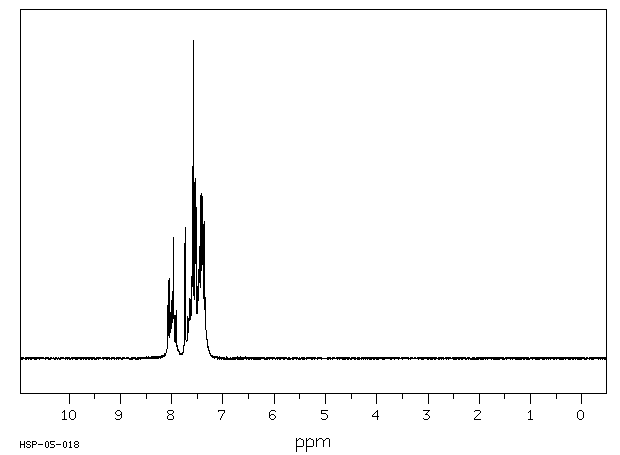
氢谱1HNMR
-
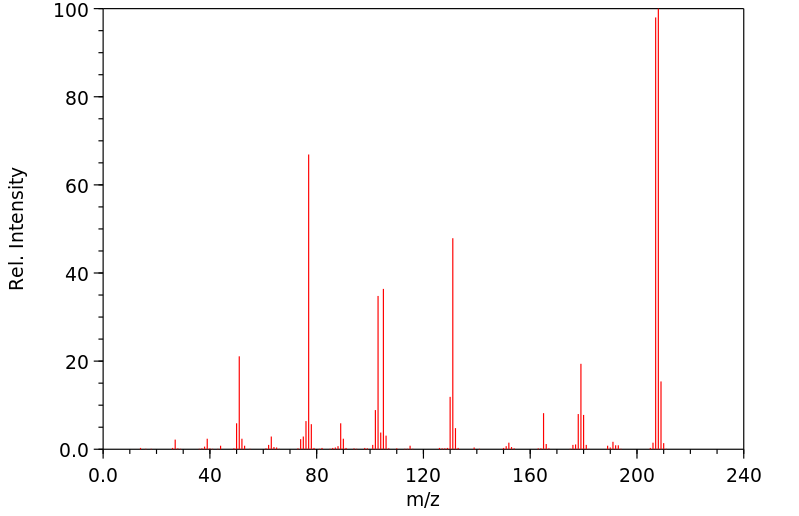
质谱MS
-
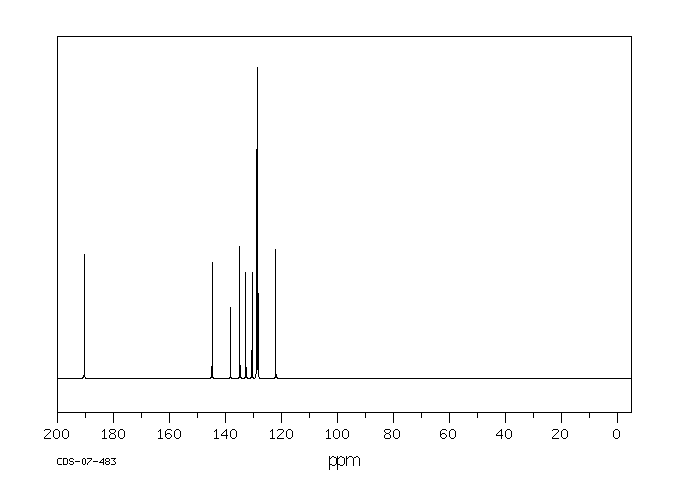
碳谱13CNMR
-
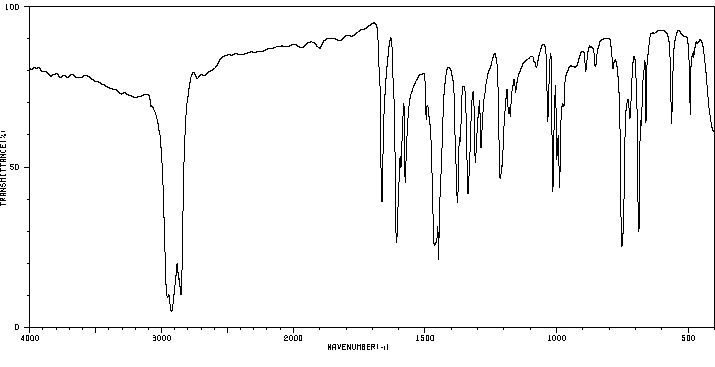
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息