5-氟-2'-脱氧脲核苷 | 50-91-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:148 °C(lit.)
-
比旋光度:35.9 º (c=1, water)
-
沸点:150 °C
-
密度:1.3751 (estimate)
-
溶解度:在水中溶解度为 100 mM,在 DMSO 中溶解度为 100 mM
-
物理描述:Inhibits DNA synthesis. (NTP, 1992)
-
颜色/状态:Crystals from butyl acetate
-
气味:ODORLESS
-
蒸汽压力:7.6X10-13 mm Hg at 25 °C (est)
-
旋光度:Specific optical rotation for D (sodium) line = +37 deg (water); +48.6 deg (DMF)
-
分解:When heated to decomposition it emits very toxic fumes of /hydrogen fluoride and nitrogen oxides/.
-
解离常数:pKa = 7.44 (imide nitrogen)
计算性质
-
辛醇/水分配系数(LogP):-1.2
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.555
-
拓扑面积:99.1
-
氢给体数:3
-
氢受体数:6
ADMET
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S22,S26,S36,S36/37/39
-
危险类别码:R22,R68,R36/37/38
-
WGK Germany:3
-
海关编码:2934999090
-
危险品运输编号:UN 2811 6.1/PG 3
-
危险类别:6.1
-
RTECS号:YU7525000
-
包装等级:III
-
危险标志:GHS06
-
危险性描述:H301
-
危险性防范说明:P261,P301+P312,P302+P352,P304+P340,P305+P351+P338
SDS
制备方法与用途
Floxuridine(脱氧氟尿苷、FUDR,NSC 27640)是一种前体药物,在体内迅速分解为5-氟尿嘧啶。该药物可用于治疗多种癌症,特别是肝脏转移的癌症。它能够抑制Poly(ADP-Ribose)聚合酶、诱导DNA损伤和凋亡,并对HSV和CMV具有抗病毒作用。
体外研究Floxuridine相较于相应的5'-O-单氨基酸酯前药,在与PEPT1的亲和力上更高。在人T淋巴细胞白血病细胞中,它与Leucovorin联用可产生协同抑制效果。Floxuridine显著抑制了[(3)H]-inosine和[(3)H]腺苷的摄取(对照组的60-70%),而其氨基酸酯前体,包括缬氨酸、苯丙氨酸、Asp和赖氨酸酯,则仅能提供较低程度的抑制效果(对照组的10-30%)。在持续36天的实验中,Floxuridine显著抑制了细胞增值超过50%,使细胞数量增加了4倍。此外,它还延长了体外培养的人眼球筋膜囊成纤维细胞的增殖时间。由于其短半衰期、陡峭的剂量反应曲线、高总清除率和高效的肝脏提取作用,Floxuridine是理想的肝动脉灌注(HAI)药物。
化学性质Floxuridine是一种白色结晶,无气味,易溶于水和甲醇,几乎不溶于氯仿和乙醚。熔点为150-151℃。比旋度为+37°至+48.6°(在DMF中测量)。其紫外吸收最大波长分别为268 nm(pH 7.2)和270 nm(pH 14)。
用途Floxuridine是一种抗肿瘤药物,适用于治疗肝癌、胃肠道癌、乳腺癌及头颈部癌。它可能引起骨髓抑制、胃肠道反应以及皮肤局部反应等副作用,并可能导致碱性磷酸酶、血清转氨酶、血清胆红质和乳酸脱氢酶水平升高。
生产方法Floxuridine的制备过程如下:首先,甲基-2-去氧-D-呋喃核糖苷进行对甲苯甲酰化处理;然后,在乙酸-盐酸条件下水解以使1位甲氧基被氯取代;之后,与1-乙酰氧汞-5-氟尿嘧啶缩合生成产物,再经水解去掉对甲苯甲酰基而得。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— [(2R,3S,5R)-5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl 3-aminooxypropanoate 1135685-99-2 C12H16FN3O7 333.273 —— 2'-deoxy-5-fluoro-5'-O-(1,3-dithian-2-yl)uridine 110238-43-2 C13H17FN2O5S2 364.419 3,5-二-o-乙酰基-5-氟-2-脱氧尿苷 2'-deoxy-3',5'-di-O-acetyl-5-fluorouridine 110522-47-9 C13H15FN2O7 330.27 —— [(2R,3S,5R)-5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl 3-aminooxy-2,2-dimethylpropanoate 1135686-00-8 C14H20FN3O7 361.327 —— 5-fluoro-O3',O5'-dipropionyl-2'-deoxy-uridine 4564-21-0 C15H19FN2O7 358.323 5-氟尿嘧啶核苷 5-fluorouridine 316-46-1 C9H11FN2O6 262.195 —— 3',5'-di-O-butanoyl-5-fluoro-2'-deoxyuridine 3343-22-4 C17H23FN2O7 386.377 —— 3',5'-O-dicaproyl-floxuridine 3415-69-8 C21H31FN2O7 442.485 2-脱氧尿苷 2'-Deoxyuridine 951-78-0 C9H12N2O5 228.205 [(2R,3S,5R)-5-(5-氟-2,4-二氧代嘧啶-1-基)-2-(辛酰氧基甲基)四氢呋喃-3-基]辛酸酯 3',5'-O-dioctanoyl-floxuridine 3415-70-1 C25H39FN2O7 498.592 —— 3',5'-di-O-benzoyl-5-fluoro-2'-deoxyuridine 2691-71-6 C23H19FN2O7 454.411 5-氟脱氧胞苷 5-fluoro-2'-deoxycytidine 10356-76-0 C9H12FN3O4 245.21 beta-胸苷 thymidine 146183-25-7 C10H14N2O5 242.232 —— [(2R,3S,5R)-5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl 2-[[(2S)-2-formamido-4-methylsulfanylbutanoyl]amino]-2-methylpropanoate 287731-60-6 C19H27FN4O8S 490.51 5-甲基尿甙 5-Methyluridine 1463-10-1 C10H14N2O6 258.231 —— [(2R,3S,5R)-5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl (2S)-2-[[(2S)-2-formamido-4-methylsulfanylbutanoyl]-methylamino]-4-methylpentanoate 287731-59-3 C22H33FN4O8S 532.59 —— (R)-1-((S)-2-Formylamino-4-methylsulfanyl-butyryl)-pyrrolidine-2-carboxylic acid (2R,3S,5R)-5-(5-fluoro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-3-hydroxy-tetrahydro-furan-2-ylmethyl ester 287731-58-2 C20H27FN4O8S 502.521 —— [(2R,3S,5R)-5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl (2S)-1-[(2S)-2-formamido-4-methylsulfanylbutanoyl]pyrrolidine-2-carboxylate 287731-57-1 C20H27FN4O8S 502.521 —— 1-<3,5-O-(tetraisopropyldisiloxane-1,3-diyl)-β-D-ribofuranosyl>-5-fluorouracil 119411-00-6 C21H37FN2O7Si2 504.703 —— 5-FdUrd-3'-O-Succ-CNGRC conjugate —— C31H44FN11O14S2 877.886 —— 3-dimethylsulfoxyimidyl-5-fluorouridine 110238-48-7 C11H16FN3O7S 353.328 2'-脱氧肌苷 2-deoxyinosine 890-38-0 C10H12N4O4 252.23 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5'-O-tosyl-5-fluoro-2'-deoxyuridine 955-24-8 C9H11FN2O5 246.195 —— 2',5'-dideoxy-5-fluorouridine 61168-97-6 C9H11FN2O4 230.196 —— 1-(2',5'-dideoxy-β-D-threo-pentofuranosyl)-5-fluorouracil 74309-06-1 C9H11FN2O4 230.196 —— 5'-O-methyl-5-fluoro-2'-deoxyuridine 24415-92-7 C10H13FN2O5 260.222 —— 5'-O-amino-2'-deoxy-5-fluorouridine 103251-31-6 C9H12FN3O5 261.21 —— 1-[(2R,4S,5R)-5-(ethoxymethyl)-4-hydroxyoxolan-2-yl]-5-fluoropyrimidine-2,4-dione 95969-43-0 C11H15FN2O5 274.249 —— 3'-O-methyl-5-fluoro-2'-deoxyuridine 24409-66-3 C10H13FN2O5 260.222 —— 3'-O-ethyl-2'-deoxy-5-fluorouridine 95969-42-9 C11H15FN2O5 274.249 —— 1-(3,5-anhydro-2-deoxy-β-D-threo-pentofuranosyl)-5-fluorouracil 144989-65-1 C9H9FN2O4 228.18 —— 2'-deoxy-5-fluoro-5'-O-(methylthiomethyl)uridine 110238-31-8 C11H15FN2O5S 306.315 —— 5'-O-butanoyl-5-fluoro-2'-deoxyuridine 7207-61-6 C13H17FN2O6 316.286 —— 5-Fluoro-2'-desoxyuridine-5'-carboxylic acid 20105-69-5 C9H9FN2O6 260.179 —— 5'-O-caproyl-floxuridine 123739-81-1 C15H21FN2O6 344.34 —— 5'-O-myristoyl-floxuridine 1111240-44-8 C23H37FN2O6 456.555 —— 5'-O-decanoyl-floxuridine 118694-12-5 C19H29FN2O6 400.447 —— 5'-O-palmitoyl-floxuridine 96733-83-4 C25H41FN2O6 484.609 —— 5'-O-lauroyl-floxuridine 118694-14-7 C21H33FN2O6 428.501 —— 5'-O-stearoyl-floxuridine 1111240-47-1 C27H45FN2O6 512.663 —— 5'-O-octanoyl-floxuridine 118694-10-3 C17H25FN2O6 372.394 —— 2'-deoxy-5-fluoro-5'-O-<(methylthio)thiocarbonyl>uridine 110238-32-9 C11H13FN2O5S2 336.365 [(2R,3S,5R)-5-(5-氟-2,4-二氧代嘧啶-1-基)-3-羟基四氢呋喃-2-基]磷酸二氢甲酯 5-fluoro-2'-deoxyuridine-5'-O-monophosphate 134-46-3 C9H12FN2O8P 326.175 —— 2'-deoxy-5-fluoro-5'-O-(methylsulfonylmethyl)uridine 149455-09-4 C11H15FN2O7S 338.314 —— 5'-O-benzyl-2'-deoxy-5-fluorouridine 95969-45-2 C16H17FN2O5 336.32 —— 5-Fluoro-2'-deoxyuridine 5'-phosphorodiamidate 74717-14-9 C9H14FN4O6P 324.206 —— 5'-O-oleoyl-FUdR 141482-25-9 C27H43FN2O6 510.647 —— 5'-azido-2',5'-dideoxy-5-fluorouridine 56512-25-5 C9H10FN5O4 271.208 —— 5'-O-(4-chlorobenzyl)-2'-deoxy-5-fluorouridine 103766-47-8 C16H16ClFN2O5 370.765 —— [(2R,3S,5R)-5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl hexadecyl hydrogen phosphate 86976-82-1 C25H44FN2O8P 550.605 —— [(2R,3S,5R)-5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl octadecyl hydrogen phosphate 86976-76-3 C27H48FN2O8P 578.659 —— 5'-O-(tert-butyldimethylsilyl)-5-fluoro-2'-deoxyuridine 129816-30-4 C15H25FN2O5Si 360.458 —— 2'-deoxy-5-fluoro-5'-O-1",3",2"-dioxaphosphacyclohex-2"-yluridine 2"-oxide 78000-60-9 C12H16FN2O8P 366.24 —— 2'-deoxy-5-fluoro-5'-O-(1,3-dithian-2-yl)uridine 110238-43-2 C13H17FN2O5S2 364.419 3,5-二-o-乙酰基-5-氟-2-脱氧尿苷 2'-deoxy-3',5'-di-O-acetyl-5-fluorouridine 110522-47-9 C13H15FN2O7 330.27 —— 3'-O-benzyl-2'-deoxy-5-fluorouridine 95969-44-1 C16H17FN2O5 336.32 —— 5-fluoro-3'-O-nitro-2'-deoxyuridine —— C9H10FN3O7 291.193 —— 1-O-octadecyl-glycerylyl-(3->5')-5-fluoro-2'-deoxyuridine —— C30H54FN2O10P 652.738 —— 2'-deoxy-5-fluoro-3'-O-(3-carboxypropanoyl)uridine 133349-29-8 C13H15FN2O8 346.269 —— Aib-FudR 502847-16-7 C13H18FN3O6 331.301 —— 3'-O-palmitoyl-floxuridine 96733-84-5 C25H41FN2O6 484.609 —— 3'-O-octanoyl-floxuridine 86977-19-7 C17H25FN2O6 372.394 —— 3-palmityloxymethyl-2'-deoxy-5-fluorouridine —— C26H45FN2O6 500.652 —— 3',5'-di-O-butanoyl-5-fluoro-2'-deoxyuridine 3343-22-4 C17H23FN2O7 386.377 —— 3'-O-pivaloyl-FUdR 1072440-12-0 C14H19FN2O6 330.313 —— 3'-O-(p-chlorobenzyl)-2'-deoxy-5-fluorouridine 103766-46-7 C16H16ClFN2O5 370.765 —— 3',5'-O-dicaproyl-floxuridine 3415-69-8 C21H31FN2O7 442.485 —— 2'-deoxy-5-fluoro-3'-O-(4-fluorobenzyl)uridine 103782-15-6 C16H16F2N2O5 354.31 3',5'-O-二棕榈酰-5-氟-2'-脱氧尿苷 3',5'-O-dipalmitoyl-floxuridine 7207-68-3 C41H71FN2O7 723.022 [(2R,3S,5R)-5-(5-氟-2,4-二氧代嘧啶-1-基)-2-(辛酰氧基甲基)四氢呋喃-3-基]辛酸酯 3',5'-O-dioctanoyl-floxuridine 3415-70-1 C25H39FN2O7 498.592 —— 3',5'-O-dimyristoyl-floxuridine 7207-67-2 C37H63FN2O7 666.915 —— 2',3'-dideoxy-5'-O-(trimethylacetyl)-5-fluorouridine 107036-60-2 C14H19FN2O5 314.314 5-氟-2',3'-二脱氧-3'-氟尿苷 3'-fluoro-2',3'-dideoxy-5-fluorouridine 41107-55-5 C9H10F2N2O4 248.186 —— 5'-O-(N-benzylthioureido)-2'-deoxy-5-fluorouridine 149455-06-1 C17H19FN4O5S 410.426 —— 3'-O-(4-methoxytetrahydropyranyl)-5-fluoro-2'-deoxyuridine 129745-68-2 C15H21FN2O7 360.339 —— 2'-deoxy-5,5",5"-trifluoro-5'-O-1",3",2"-dioxaphosphacyclohex-2"-yluridine 2"-oxide 88946-06-9 C12H14F3N2O8P 402.221 —— 5-fluoro-1-[(2R,4S,5R)-5-(hydroxymethyl)-4-(naphthalen-2-ylmethoxy)oxolan-2-yl]pyrimidine-2,4-dione 179744-28-6 C20H19FN2O5 386.38 —— β-D-2'-deoxy-3',5'-di-O-mesyl-5-fluorouridine 7239-42-1 C11H15FN2O9S2 402.379 —— 3'-O-<(tert-butyl)dimethylsilyl>-2'-deoxy-5-fluorouridine 120957-59-7 C15H25FN2O5Si 360.458 —— 3'-O-(3-chlorobenzyl)-2'-deoxy-5-fluorouridine 103767-16-4 C16H16ClFN2O5 370.765 2'-脱氧-5-氟-3-(2-四氢呋喃基)尿苷 2'-deoxy-5-fluoro-3-(2-tetrahydrofuranyl)uridine 103782-09-8 C13H17FN2O6 316.286 —— 5'-O-benzoyl-FUdR 1006381-74-3 C16H15FN2O6 350.303 —— 2'-deoxy-3'-O-(p-methoxybenzyl)-5-fluorouridine 103766-50-3 C17H19FN2O6 366.346 —— 5'-O-β-D-galactosyl-floxuridine 163218-40-4 C15H21FN2O10 408.338 —— 3-amino-2'-deoxy-5-flurouridine 110238-12-5 C9H12FN3O5 261.21 —— 5-fluoro-1-[(2R,4S,5R)-5-(hydroxymethyl)-4-[[4-(trifluoromethyl)phenyl]methoxy]oxolan-2-yl]pyrimidine-2,4-dione 150109-62-9 C17H16F4N2O5 404.318 —— N-<1,2,5-trideoxy-1β-(5-fluoro-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-1-yl)-D-ribofuranos-5-yl>succinamic acid —— C13H16FN3O7 345.284 —— 3'-O-(2-chlorobenzyl)-2'-deoxy-5-fluorouridine 103767-17-5 C16H16ClFN2O5 370.765 —— 3-n-heptanoyloxymethyl-2'-deoxy-5-fluorouridine 173531-00-5 C17H25FN2O7 388.393 —— 3-n-octanoyloxymethyl-2'-deoxy-5-fluorouridine 161632-23-1 C18H27FN2O7 402.42 —— N-butyl-N'-<1,2,5-trideoxy-1β-(5-fluoro-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-1-yl)-D-ribofuranose-5-yl>maleamide —— C17H23FN4O6 398.391 —— 2'-deoxy-5-fluoro-3'-O-(1-naphthylmethyl)uridine 103767-18-6 C20H19FN2O5 386.38 —— methyl N-<1,2,5-trideoxy-1β-(5-fluoro-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-1-yl)-D-ribofuranos-5-yl>succinamate 149063-95-6 C14H18FN3O7 359.311 —— 5-fluoro-1-[(2R,4S,5R)-5-(methoxymethyl)-4-[(4-methoxyphenyl)methoxy]oxolan-2-yl]pyrimidine-2,4-dione 1053613-37-8 C18H21FN2O6 380.373 —— 2'-deoxy-3'-O-(4-dimethylaminobenzyl)-5-fluorouridine 103792-37-6 C18H22FN3O5 379.388 —— 1-((4aR,6R,7aS)-2-Dimethylamino-tetrahydro-furo[3,2-d][1,3,2]dioxaphosphinin-6-yl)-5-fluoro-1H-pyrimidine-2,4-dione 125968-39-0 C11H15FN3O5P 319.229 —— N-<1,2,5-trideoxy-1β-(5-fluoro-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-1-yl)-D-ribofuranos-5-yl>maleamic acid —— C13H14FN3O7 343.268 —— 5-fluoro-1-[(2R,4S,5R)-4-[(4-methoxyphenyl)methoxy]-5-(phenylmethoxymethyl)oxolan-2-yl]pyrimidine-2,4-dione 1053249-03-8 C24H25FN2O6 456.471 —— bis<(pivaloyloxy)methyl> 2'-deoxy-5-fluorouridine 5'-phosphate —— C21H32FN2O12P 554.463 —— 1-[(2R,4S,5R)-5-(ethoxymethyl)-4-[(4-methoxyphenyl)methoxy]oxolan-2-yl]-5-fluoropyrimidine-2,4-dione 1053384-21-6 C19H23FN2O6 394.4 —— 2'-deoxy-5-fluoro-5'-O-(benzo<1,3>dithiol-2-yl)uridine 110238-34-1 C16H15FN2O5S2 398.436 —— ethyl N-[1,2,5-trideoxy-1β-(5-fluoro-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-1-yl)-D-ribofuranos-5-yl]succinamate 149063-97-8 C15H20FN3O7 373.338 —— 5'-O-L-prolyl-floxuridine 675829-80-8 C14H18FN3O6 343.312 —— 3'-O-benzyl-5'-O-chloroacetyl-2'-deoxy-5-fluorouridine 103782-11-2 C18H18ClFN2O6 412.802 —— 1-[(2R,4S,5R)-4-[tert-butyl(dimethyl)silyl]oxy-5-(ethoxymethyl)oxolan-2-yl]-5-fluoropyrimidine-2,4-dione 1053259-12-3 C17H29FN2O5Si 388.512 —— 2'-deoxy-5-fluorouridine 5'-O-triphosphate 2710-64-7 C9H14FN2O14P3 486.135 —— 5'-O-(2-morpholinopropionyl)-2'-deoxy-5-fluorouridine 80667-47-6 C16H22FN3O7 387.365 —— 1-(2'-deoxy-β-D-ribofuranosyl)-5-fluorouracil-5'-<2-(2-oxo-1,3,2-oxazaphosphorinane)> 77086-92-1 C12H17FN3O7P 365.255 5-氟-1-[4-羟基-5-[三(苯基)甲氧基甲基]四氢呋喃-2-基]嘧啶-2,4-二酮 5'-O-trityl-5-fluoro-2'-deoxyuridine 10343-71-2 C28H25FN2O5 488.515 —— 1-(5-O-Trityl-2-deoxy-β-D-lyxofuranosyl)-5-fluorouracil 103285-11-6 C28H25FN2O5 488.515 —— 3'-O-benzoyl-5-fluoro-2'-deoxyuridine 1995-83-1 C16H15FN2O6 350.303 —— 5'-O-acetyl-3'-deoxy-3',5-difluoro-2'-deoxyuridine 87412-13-3 C11H12F2N2O5 290.223 —— 2'-deoxy-5-fluoro-5'-O-(p-toluenesulfonyl)uridine 10054-41-8 C16H17FN2O7S 400.385 —— 2'-deoxy-5-fluoro-3'-O-(2-pyridyl)uridine 114667-93-5 C14H14FN3O5 323.281 —— 5'-chloro-2',5'-dideoxy-5-fluorouridine 3'-O-phosphate 736907-94-1 C9H11ClFN2O7P 344.621 —— 1-(3-azido-2,3-dideoxy-β-D-erythro-pentofuranosyl)-5-fluorouracil 87190-74-7 C9H10FN5O4 271.208 —— 3',5'-di-O-benzoyl-5-fluoro-2'-deoxyuridine 2691-71-6 C23H19FN2O7 454.411 —— t-Boc-Aib-FudR 287731-67-3 C18H26FN3O8 431.418 —— 2'-deoxy-5-fluoro-5'-O-(5-chlorobenzo<1,3>dithiol-2-yl)uridine 149455-07-2 C16H14ClFN2O5S2 432.881 —— 2'-deoxy-5-fluoro-5'-O-(5-methylbenzo<1,3>dithiol-2-yl)uridine 149455-08-3 C17H17FN2O5S2 412.463 —— 1-[(2R,4S,5R)-4-ethoxy-5-(trityloxymethyl)oxolan-2-yl]-5-fluoropyrimidine-2,4-dione 1054726-96-3 C30H29FN2O5 516.569 —— 1-[(2R,4S,5R)-5-[[tert-butyl(dimethyl)silyl]oxymethyl]-4-phenylmethoxyoxolan-2-yl]-5-fluoropyrimidine-2,4-dione 150109-59-4 C22H31FN2O5Si 450.582 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:5-氟-2'-脱氧脲核苷 在 4-二甲氨基吡啶 、 水 、 N,N'-二环己基碳二亚胺 、 三氟乙酸 作用下, 以 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 28.0h, 生成 3',5'-di-O-D-valyl-floxuridine trifluoroacetate参考文献:名称:氟尿苷的氨基酸酯前药:合成及结构,立体化学和酯化的位置对水解速率的影响。摘要:目的合成氟尿苷(FUdR)的氨基酸酯前药,并研究结构,立体化学和蛋白质的酯化部位对这些前药在Caco-2细胞匀浆中水解速率的影响。方法采用既定程序合成FUdR的氨基酸酯前药。在人腺癌细胞系(Caco-2)匀浆和pH 7.4磷酸盐缓冲液中评估了前药水解的动力学。结果合成并表征了以脯氨酸,L-缬氨酸,D-缬氨酸,L-苯丙氨酸和D-苯丙氨酸为原基的FUdR的3'-单酯,5'-单酯和3',5'-二酯前药。在Caco-2细胞匀浆中,L-氨基酸酯前药的水解速度是相应D-氨基酸酯前药的10到75倍。Pro和Phe酯前药的水解速度比相应的Val酯前药快得多(3至30倍)。此外,5'-单酯前药的水解明显快于3',5'-二酯前药的水解(3倍)。结论成功合成了新型的FUdR氨基酸酯前药。此处显示的结果清楚地表明,Caco-2细胞匀浆中FUdR前药的活化速率受该结构的结构,立体化学和酯化部位的影响。最后,5'-VDOI:10.1023/a:1025745824632
-
作为产物:描述:参考文献:名称:5-取代的2'-脱氧尿苷异头体的立体选择性合成摘要:研究了5-取代的2,4-双(三甲基甲硅烷氧基)嘧啶与3,5-双(Op-氯苯甲酰基)-2-脱氧-α-D-呋喃核糖基氯的取代反应。在对硝基苯酚的存在下,β端基异构体立体选择性地形成,而有机碱的加入导致α端基异构体的立体选择性形成。反应的立体选择性取决于二甲硅烷基嘧啶的 5 位取代基、添加剂和每种试剂的浓度。5-取代的2'-脱氧-尿苷的α和β异头通过5-取代3',5'-二-O-(对-氯苯甲酰基)-2'-脱氧尿苷的α和β异头脱酰化合成。DOI:10.1246/bcsj.60.2073
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
Heterocyclic derivatives for the treatment of cancer and other proliferative diseases申请人:——公开号:US20020143182A1公开(公告)日:2002-10-03The invention relates to certain heterocyclic compounds useful for the treatment of cancer and other diseases, having the Formula (I): 1 wherein: (a) m is an integer 0 or 1; (b) R 12 is an alkyl, a substituted alkyl, a cycloalkyl, a substituted cycloalkyl, a heterocyclic, a substituted heterocyclic, a heteroaryl, a substituted heteroaryl, an aryl or a substituted aryl residue; (c) Ar 3 is an aryl, a substituted aryl, a heteroaryl or a substituted heteroaryl residue; (d) Ar 4 is an aryl, a substituted aryl, a heteroaryl or a substituted heteroaryl residue; (e) R 5 is hydrogen, hydroxy, alkyl or substituted alkyl; (f) - - - - - represents a bond present or absent; and (g) W, X, Y and Z are independently or together C(O)—, C(S), S, O, or NH; or a pharmaceutically acceptable salt thereof.该发明涉及某些对治疗癌症和其他疾病有用的杂环化合物,其具有以下式(I): 1 其中: (a) m是整数0或1; (b) R12是烷基,取代烷基,环烷基,取代环烷基,杂环基,取代杂环基,杂芳基,取代杂芳基,芳基或取代芳基残基; (c) Ar3是芳基,取代芳基,杂芳基或取代杂芳基残基; (d) Ar4是芳基,取代芳基,杂芳基或取代杂芳基残基; (e) R5是氢,羟基,烷基或取代烷基; (f) - - - - - 代表存在或不存在的键;以及 (g) W、X、Y和Z独立或一起是C(O)、C(S)、S、O或NH;或其药学上可接受的盐。
-
[EN] COMPOUNDS AND COMPOSITIONS COMPRISING CDK INHIBITORS AND METHODS FOR THE TREATMENT OF CANCER<br/>[FR] COMPOSÉS ET COMPOSITIONS COMPRENANT DES INHIBITEURS DES CDK ET MÉTHODES DE TRAITEMENT DU CANCER申请人:UNIV GEORGIA STATE RES FOUND公开号:WO2010129858A1公开(公告)日:2010-11-11Disclosed herein are compounds suitable for use as antitumor agents, methods for treating cancer wherein the disclosed compounds are used in making a medicament for the treatment of cancer, methods for treating a tumor comprising, administering to a subject a composition comprising one or more of the disclosed cytotoxic agents, and methods for preparing the disclosed antitumor agents.
-
Cobalamin conjugates for anti-tumor therapy申请人:Weinshenker M. Ned公开号:US20050054607A1公开(公告)日:2005-03-10The present invention provides a cobalamin-drug conjugate suitable for the treatment of tumor related diseases. Cobalamin is indirectly covalently bound to an anti-tumor drug via a cleavable linker and one or more optional spacers. Cobalamin is covalently bound to a first spacer or the cleavable linker via the 5′-OH of the cobalamin ribose ring. The drug is bound to a second spacer of the cleavable linker via an existing or added functional group on the drug. After administration, the conjugate forms a complex with transcobalamin (any of its isoforms). The complex then binds to a receptor on a cell membrane and is taken up into the cell. Once in the cell, an intracellular enzyme cleaves the conjugate thereby releasing the drug. Depending upon the structure of the conjugate, a particular class or type of intracellular enzyme affects the cleavage. Due to the high demand for cobalamin in growing cells, tumor cells typically take up a higher percentage of the conjugate than do normal non-growing cells. The conjugate of the invention advantageously provides a reduced systemic toxicity and enhanced efficacy as compared to a corresponding free drug.本发明提供了一种适用于治疗肿瘤相关疾病的钴胺素-药物结合物。钴胺素通过可切割的连接剂间接共价结合到抗肿瘤药物上,还可以通过一个或多个可选的间隔物。钴胺素通过其核糖环的5'-OH与第一间隔物或可切割连接剂共价结合。药物通过其现有或添加的功能基团与可切割连接剂的第二间隔物结合。在给药后,结合物与转钴胺素(其任何同工异构体)形成复合物。然后,该复合物结合到细胞膜上的受体并被细胞摄取。一旦进入细胞,细胞内酶将切割结合物,从而释放药物。根据结合物的结构,特定类别或类型的细胞内酶影响切割。由于生长细胞对钴胺素的需求量较高,肿瘤细胞通常摄取结合物的比例高于正常非生长细胞。本发明的结合物与相应的游离药物相比,具有较低的全身毒性和增强的疗效。
-
[EN] INHIBITORS OF BRUTON'S TYROSINE KINASE<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON申请人:BIOCAD JOINT STOCK CO公开号:WO2018092047A1公开(公告)日:2018-05-24The present invention relates to a new compound of formula I: or pharmaceutically acceptable salt, solvate or stereoisomer thereof, wherein: V1 is C or N, V2 is C(R2) or N, whereby if V1 is C then V2 is N, if V1 is C then V2 is C(R2), or if V1 is N then V2 is C(R2); each n, k is independently 0, 1; each R2, R11 is independently H, D, Hal, CN, NR'R", C(O)NR'R", C1-C6 alkoxy; R3 is H, D, hydroxy, C(O)C1-C6 alkyl, C(O)C2-C6 alkenyl, C(O)C2-C6 alkynyl, C1-C6 alkyl; R4 is H, Hal, CN, CONR'R", hydroxy, C1-C6 alkyl, C1-C6 alkoxy; L is CH2, NH, O or chemical bond; R1 is selected from the group of the fragments, comprising: Fragment 1, Fragment 2, Fragment 3 each A1, A2, A3, A4 is independently CH, N, CHal; each A5, A6, A7, A8, A9 is independently C, CH or N; R5 is H, CN, Hal, CONR'R", C1-C6 alkyl, non-substituted or substituted by one or more halogens; each R' and R" is independently selected from the group, comprising H, C1-C6 alkyl, C1-C6 cycloalkyl, aryl; R6 is selected from the group: [formula II] each R7, R8, R9, R10 is independently vinyl, methylacetylenyl; Hal is CI, Br, I, F, which have properties of inhibitor of Bruton's tyrosine kinase (Btk), to pharmaceutical compositions containing such compounds, and their use as pharmaceuticals for treatment of diseases and disorder.本发明涉及一种新的化合物,其化学式为I:或其药学上可接受的盐、溶剂化合物或立体异构体,其中:V1为C或N,V2为C(R2)或N,如果V1为C,则V2为N,如果V1为C,则V2为C(R2),或者如果V1为N,则V2为C(R2);每个n,k独立地为0或1;每个R2,R11独立地为H,D,Hal,CN,NR'R",C(O)NR'R",C1-C6烷氧基;R3为H,D,羟基,C(O)C1-C6烷基,C(O)C2-C6烯基,C(O)C2-C6炔基,C1-C6烷基;R4为H,Hal,CN,CONR'R",羟基,C1-C6烷基,C1-C6烷氧基;L为CH2,NH,O或化学键;R1从包括的片段组中选择:片段1,片段2,片段3,每个A1,A2,A3,A4独立地为CH,N,CHal;每个A5,A6,A7,A8,A9独立地为C,CH或N;R5为H,CN,Hal,CONR'R",C1-C6烷基,未取代或被一个或多个卤素取代;每个R'和R"独立地从包括H,C1-C6烷基,C1-C6环烷基,芳基的组中选择;R6从组中选择:[化学式II]每个R7,R8,R9,R10独立地为乙烯基,甲基乙炔基;Hal为CI,Br,I,F,具有布鲁顿酪氨酸激酶(Btk)抑制剂的性质,以及含有这种化合物的药物组合物,以及它们作为治疗疾病和紊乱的药物的用途。
表征谱图
-
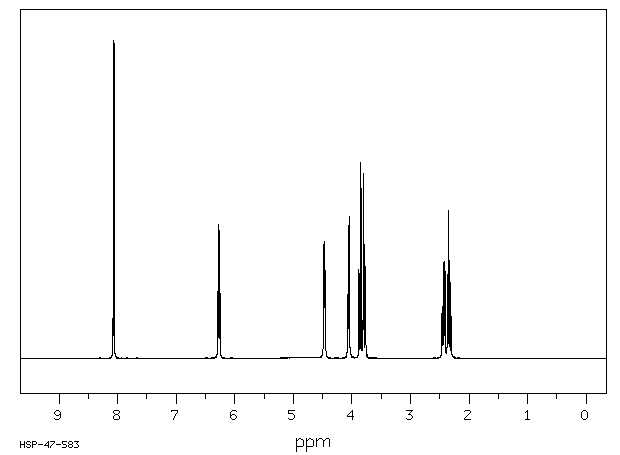
氢谱1HNMR
-
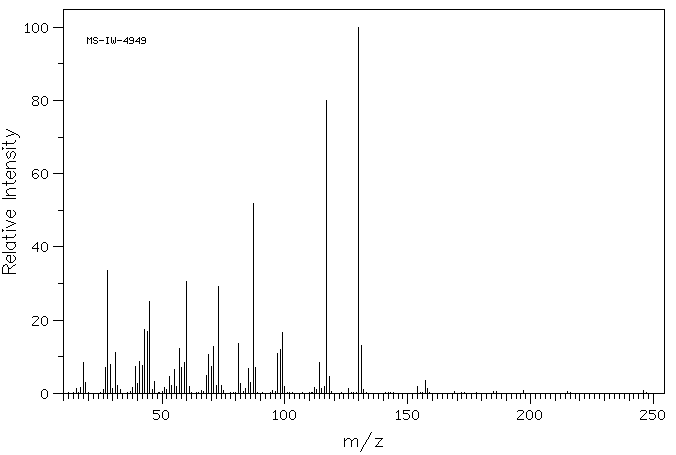
质谱MS
-
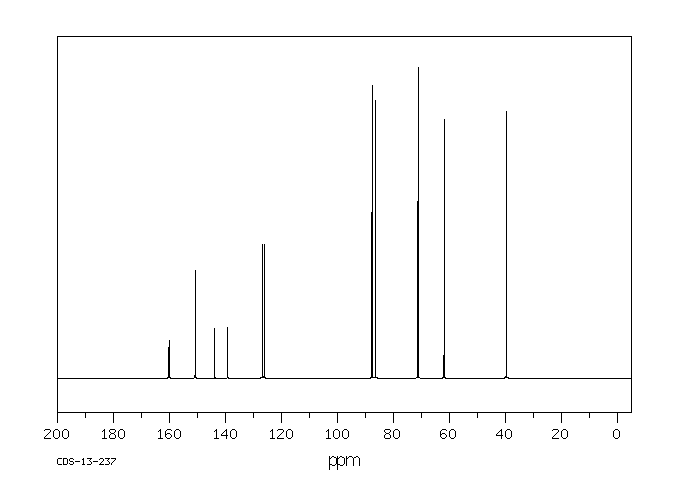
碳谱13CNMR
-
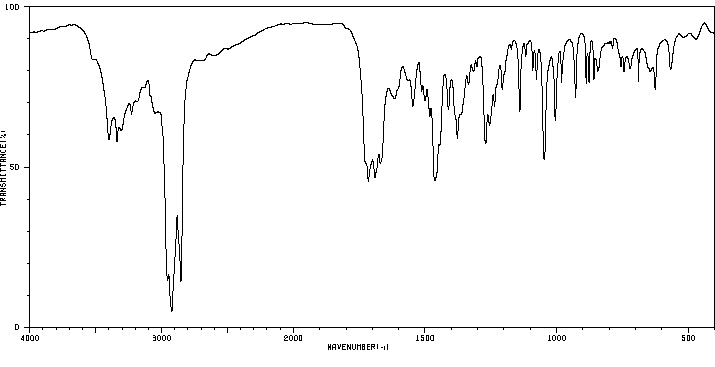
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息