表雄酮 | 481-29-8
物质功能分类
分子结构分类
中文名称
表雄酮
中文别名
表雄甾酮;异雄甾酮;5α-雄甾-3β-醇-17-酮;异雄酮,异雄酮
英文名称
Epiandrosterone
英文别名
3β-hydroxy-5α-androstan-17-one;trans-androsterone;5α-androstan-3β-ol-17-one;(3S,5S,8R,9S,10S,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,14,15,16-tetradecahydrocyclopenta[a]phenanthren-17-one
CAS
481-29-8
化学式
C19H30O2
mdl
——
分子量
290.446
InChiKey
QGXBDMJGAMFCBF-LUJOEAJASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172-174 °C
-
比旋光度:91 º (c=1, CH3OH)
-
沸点:372.52°C (rough estimate)
-
密度:1.0320 (rough estimate)
-
溶解度:不溶于水;乙醇中≥12.35 mg/mL; ≥28.27 mg/mL,溶于 DMSO
-
LogP:3.751
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:21
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.947
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S22,S24/25
-
WGK Germany:3
-
海关编码:2937290090
-
危险品运输编号:NONH for all modes of transport
SDS
制备方法与用途
生物活性 Epiandrosterone (3β-androsterone, EpiA) 是一种类固醇激素,具有微弱的雄激素活性,并且是DHEA的一种天然代谢产物。
靶点
- AR
- ER
体外研究 Epiandrosterone是通过5α-还原酶从DHEA自然转化而来的。它在外周组织中生成并释放到血液循环中,最终通过尿液排出体外。尽管Epiandrosterone具有微弱的雄激素活性,但它广泛被认为能抑制磷酸戊糖途径(PPP),从而降低细胞内NADPH水平。此外,研究发现Epiandrosterone可以减少NO-诱导的肺动脉松弛,并且在隔离肺组织中,它能够抑制血管紧张素II和缺氧引起的血管收缩,同时还能使KCl预收缩的孤立肺动脉舒张。
化学性质 针状结晶(氯仿/己烷),熔点176-177.5℃(174-175℃,161-162℃)。[α]20/D+44.4(C0.27氯仿);加热时有麝香气味。
用途 用于甾体激素类药物的生产。
生产方法 通过使用钯炭催化氢化5-雄甾烯-3β-醇-17-酮3-醋酸酯,得到雄甾烷-3β-醇-17酮3-醋酸酯;随后用水解反应制得Epiandrosterone。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 雄酮 cis-androsterone 53-41-8 C19H30O2 290.446 别孕烯醇酮 isopregnanolone 516-55-2 C21H34O2 318.5 5a-雄甾烷二酮 androstanedione 846-46-8 C19H28O2 288.43 醋酸去氢表雄酮 3β-acetoxy-5α-androstan-17-one 1239-31-2 C21H32O3 332.483 乙酸雄甾酮 androsterone acetate 1164-95-0 C21H32O3 332.483 雄诺龙 Stanolone 521-18-6 C19H30O2 290.446 —— (3β,5α)-3-[(methylsulfonyl)oxy]androstan-17-one 19646-53-8 C20H32O4S 368.538 去氢表雄酮 dehydroepiandrosterone 53-43-0 C19H28O2 288.43 5alpha-雄烷二醇 5-androgen-3,17-diol 571-20-0 C19H32O2 292.462 —— 17-oxo-5α-androstan-3β-yl benzoate 6696-39-5 C26H34O3 394.554 —— 5α-cholestan-3β-yl acetate 1255-88-5 C29H50O2 430.715 —— 3β-acetoxy-5α-stigmastane 2364-21-8 C31H54O2 458.769 —— campestanyl acetate 4356-09-6 C30H52O2 444.742 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 雄酮 cis-androsterone 53-41-8 C19H30O2 290.446 —— 3α-hydroxy-2β-methylandrostan-17-one —— C20H32O2 304.473 —— 3β-Hydroxy-5α-androstan-7,17-dion 49643-99-4 C19H28O3 304.43 —— 3β-hydroxypregnan-16-one 1096-41-9 C21H34O2 318.5 —— 2β,3α-dihydroxy-5α-androst-17-one 83808-48-4 C19H30O3 306.445 —— 3β-methoxy-5α-androstan-17-one 7680-12-8 C20H32O2 304.473 —— 3β,7α-Dihydroxy-5α-androstan-17-on 25848-68-4 C19H30O3 306.445 —— 3β,7β-Dihydroxy-5α-androstan-17-on 25848-69-5 C19H30O3 306.445 别孕烯醇酮 isopregnanolone 516-55-2 C21H34O2 318.5 —— 2α,3α-epoxy-5α-androstan-17-one 965-67-3 C19H28O2 288.43 5alpha-雄甾烷-3beta-醇-16-酮 3β-hydroxy-5α-androstan-16-one 571-51-7 C19H30O2 290.446 —— 3β,6α-Dihydroxy-5α-androstan-17-on 14895-71-7 C19H30O3 306.445 —— 3β,16α-dihydroxy-5α-androstan-17-one 1159-67-7 C19H30O3 306.445 —— 3β,11α-Dihydroxy-5α-androstan-17-on 25848-75-3 C19H30O3 306.445 —— (3β,5α,11β)-3,11-dihydroxyandrostan-17-one 116698-46-5 C19H30O3 306.445 —— 17-Oxo-2.3-seco-5α-androstan-2.3-dicarbonsaeure 1165-38-4 C19H28O5 336.428 —— 6α-hydroxyandrostane-3,17-dione 49643-95-0 C19H28O3 304.43 —— 11α-hydroxy-5α-androstan-3,17-dione 29907-31-1 C19H28O3 304.43 —— (3S,5S,8R,9S,10S,13S,14S)-3-(methoxymethyl ether)-10,13-dimethylhexadecahydro-17H-cyclopenta[a]phenanthren-17-one 66211-84-5 C21H34O3 334.499 —— trichiliasterone A 183172-90-9 C21H32O3 332.483 —— (1S,2S,8R,11R,12S,16S)-2,16-dimethyltetracyclo[9.7.0.02,8.012,16]octadecan-15-one 1382470-08-7 C20H32O 288.473 (5alpha)-雄甾烷-17-酮 5alpha-androstan-17-one 963-74-6 C19H30O 274.447 5a-雄甾烷二酮 androstanedione 846-46-8 C19H28O2 288.43 —— 3β-trimethylsilyloxy-5α-H-androstan-17-one 10426-95-6 C22H38O2Si 362.628 醋酸去氢表雄酮 3β-acetoxy-5α-androstan-17-one 1239-31-2 C21H32O3 332.483 乙酸雄甾酮 androsterone acetate 1164-95-0 C21H32O3 332.483 —— 3β,12β-Dihydroxy-5α-androstan-17-on 848-63-5 C19H30O3 306.445 —— 5α-androstane-3,7,17-trione 4147-15-3 C19H26O3 302.414 —— epiandrosterone 3-butanoate 15253-56-2 C23H36O3 360.537 3,3-二甲氧基-5Α-雄烷-17-酮 3,3-(dimethoxy)-5α-androstan-17-one 3591-19-3 C21H34O3 334.499 —— 17-Oxo-2.3-seco-5α-androstan-2.3-dicarbonsaeure-dimethylester 1169-77-3 C21H32O5 364.482 —— Androsterone-3β-chloroformate 15233-27-9 C20H29ClO3 352.901 —— 3α,4α-epoxy-5α-androstan-17-one —— C19H28O2 288.43 —— 5α-androst-3,6,17-trione 2243-05-2 C19H26O3 302.414 —— epiandrosterone 3-hexadecanoate 54614-67-4 C35H60O3 528.86 —— (3S,5S,8R,9S,10S,13S,14S)-10,13-dimethyl-17-oxohexadecahydro-1H-cyclopenta[a]phenanthren-3-yl 4-methylpentanoate —— C25H40O3 388.591 —— 3β-Acetoxy-7,17-dioxo-5α-androstan 13258-27-0 C21H30O4 346.467 —— 5α-Androstan-1.3.17-trion 4171-02-2 C19H26O3 302.414 16-溴表雄酮 16α-bromo-3β-hydroxy-5α-androstan-17-one 28507-02-0 C19H29BrO2 369.342 —— 16α-fluoro-3β-hydroxy-5α-androstan-17-one 80724-82-9 C19H29FO2 308.436 —— (1S,2S,8S,11R,12S,16S)-2,16-dimethyl-5-oxatetracyclo[9.7.0.02,8.012,16]octadecane-6,15-dione 1315357-90-4 C19H28O3 304.43 —— Epi-Androsterone sulfate 977-35-5 C19H30O5S 370.51 —— 5α-androstane-3,12,17-trione 4171-01-1 C19H26O3 302.414 雄诺龙 Stanolone 521-18-6 C19H30O2 290.446 甲氢睾酮 mesterolone 1424-00-6 C20H32O2 304.473 —— (3β,5α)-3-[(methylsulfonyl)oxy]androstan-17-one 19646-53-8 C20H32O4S 368.538 —— (3α,4β,5α)-3-hydroxy-4-methoxyandrostan-17-one 1516884-15-3 C20H32O3 320.472 —— (5α,20S)-3-oxopregnane-20-carboxylic acid 115208-40-7 C22H34O3 346.51 氢化黄体酮 dihydroprogesterone 566-65-4 C21H32O2 316.484 —— 3β-chloro-5α-androstan-17-one 20612-47-9 C19H29ClO 308.892 —— 3α-Chlor-5α-androstan-17-on 51104-91-7 C19H29ClO 308.892 罗库溴铵杂质1263 (3R,5S,8R,9S,10S,13S,14S)-3-bromo-10,13-dimethylhexadecahydro-17H-cyclopenta[a]phenanthren-17-one 53512-66-6 C19H29BrO 353.343 —— (3S,5S,8R,9S,10S,13S,14S)-3-bromo-10,13-dimethylhexadecahydro-17H-cyclopenta[a]phenanthren-17-one 58507-05-4 C19H29BrO 353.343 —— 5α-androstan-17-on-3β-yl diazoacetate 385449-67-2 C21H30N2O3 358.481 —— 3α-amino-5α-androstan-17-one 21370-87-6 C19H31NO 289.461 —— (3β,5α)-3-aminoandrostan-17-one —— C19H31NO 289.461 —— 3α-fluoro-5αH-androstan-17-one 1156-86-1 C19H29FO 292.437 —— 3β-fluoro-5αH-androstan-17-one 361-80-8 C19H29FO 292.437 —— methyl 3β-hydroxy-17-oxo-5α-androstane-16-carboxylate 13542-28-4 C21H32O4 348.483 —— (1S,2R,8R,11R,12S,16S)-2,16-dimethyl-6-oxatetracyclo[9.7.0.02,8.012,16]octadecane-5,15-dione 1161927-13-4 C19H28O3 304.43 —— 3β-<(tetrahydro-2H-pyran-2-yl)oxy>-5α-androstan-17-one 1458-81-7 C24H38O3 374.564 5alpha-雄甾-2-烯-17-酮 5α-androst-2-en-17-one 963-75-7 C19H28O 272.431 —— 3β-hydroxy-16-(hydroxymethylene)-5α-androstan-17-one 6174-76-1 C20H30O3 318.456 —— 11α,17β-dihydroxy-5α-androstan-3-one 25788-56-1 C19H30O3 306.445 5Α-雄甾烷-3Β-醇 5α-androstan-3β-ol 1224-92-6 C19H32O 276.462 美雄诺龙 mestanolone 521-11-9 C20H32O2 304.473 —— 5α-pregnan-3β-ol 4352-06-1 C21H36O 304.516 —— (3S,10S,13S)-3-((tert-butyldimethylsilyl)oxy)-10,13-dimethyl-1,2,3,4,7,8,9,10,11,12,13,14,15,16-tetradecahydro-17H-cyclopenta-[a]phenanthren-17-one 57711-44-1 C25H44O2Si 404.709 —— 17β-hydroxy-17α-ethyl-5α-androstan-3-one 20112-30-5 C21H34O2 318.5 (5S,8S,9S,10S,13R,14S)-10,13-二甲基-2,4,5,6,7,8,9,11,12,14,15,17-十二氢-1H-环戊并[a]菲-3,16-二酮 5α-Androstan-3,16-dion 1035-71-8 C19H28O2 288.43 5-雄甾-3-烯-17-酮 5α-androst-3-en-17-one 14935-81-0 C19H28O 272.431 —— androstan-3-one 118371-41-8 C19H30O 274.447 —— (5α)-pregnan-3-one 14778-11-1 C21H34O 302.5 —— 16α-bromo-5α-androstan-17-one 5987-29-1 C19H29BrO 353.343 —— (6aS,6bS,8aS,11aS,11bR,13aS)-6a,8a-dimethylhexadecahydro-3H-cyclopenta[5,6]naphtho[1,2-d]azocine-3,9(2H)-dione 1382474-85-2 C20H31NO2 317.472 —— 3α-azido-5α-androstan-17-one 7795-05-3 C19H29N3O 315.459 —— (20S)-3β-<(tert-butyldimethylsilyl)oxy>-5α-cholestan-15,23-dione —— C33H58O3Si 530.907 —— (20R)-3β-<(tert-butyldimethylsilyl)oxy>-5α-cholestane-15,23-dione 224448-07-1 C33H58O3Si 530.907 —— 3α-hydroxy-5α-androst-9(11)-en-17-one 14043-47-1 C19H28O2 288.43 (5alpha)-17,21-二羟基孕甾烷e-3,20-二酮 17α-21-dihydroxy-5-α-pregnane-3,20-dione 312-99-2 C21H32O4 348.483 —— pregnane-3,20-diol 38270-91-6 C21H36O2 320.516 —— 3-aza-A-homo-5α-androstane-4,17-dione 118204-35-6 C19H29NO2 303.445 1-雄烯二酮 5α-androst-1-ene-3,17-dione 571-40-4 C19H26O2 286.414 5alpha-雄烷二醇 5-androgen-3,17-diol 571-20-0 C19H32O2 292.462 (3beta,5beta,17alpha)-雄甾烷-3,17-二醇 5α-androstane-3β,17α-diol 5856-11-1 C19H32O2 292.462 —— dihydroandrosterone 62356-64-3 C19H32O2 292.462 —— 17-oxo-5α-androstan-3β-yl benzoate 6696-39-5 C26H34O3 394.554 —— 3α-hydroxy-5α-androstan-17-one 3-benzoate 5953-69-5 C26H34O3 394.554 —— (3S,5S,8R,9S,10S,13S,14R)-3-(methoxymethoxy)-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,14-dodecahydrocyclopenta[a]phenanthren-17-one 210641-52-4 C21H32O3 332.483 —— (3S,5S,8R,9S,10S,13S,14S)-3-(methoxymethyl ether)-10,13-dimethyl-1,2,3,4,5,6,7,8,9,10,11,12,13,14-tetradecahydro-17H-cyclopenta[a]phenanthren-17-one 210641-49-9 C21H32O3 332.483 —— 3β-acetoxy-5α-androstane 1236-13-1 C21H34O2 318.5 —— N-[(3β,5α)-17-oxoandrostan-3-yl]acetamide 4410-61-1 C21H33NO2 331.499 —— 3α-Acetamino-5α-androstan-17-on 4410-61-1 C21H33NO2 331.499 —— 3α,11β-Dihydroxy-9α-fluoro-5α-androstan-17-one 2063-66-3 C19H29FO3 324.436 (5R,8R,9S,10S,13S,14S,17S)-10,13-二甲基-2,3,4,5,6,7,8,9,11,12,14,15,16,17-十四氢-1H-环戊并[a]菲-17-醇 (5R,8R,9S,10S,13S,14S,17S)-10,13-Dimethyl-hexadecahydro-cyclopenta[a]phenanthren-17-ol 1225-43-0 C19H32O 276.462 —— (2S)-2-[(3S,5S,8R,9S,10S,13S,14S,17R)-3-[tert-butyl(dimethyl)silyl]oxy-10,13-dimethyl-16-oxo-1,2,3,4,5,6,7,8,9,11,12,14,15,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]propanenitrile 216572-49-5 C28H47NO2Si 457.772 —— 4-aza-A-homo-5α-androstan-3,17-dione 99800-97-2 C19H29NO2 303.445 —— 17α-ethynyldihydrotestosterone 13611-97-7 C21H30O2 314.468 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:表雄酮 在 chromium(VI) oxide 、 乙醚 、 copper(II) sulfate 、 溶剂黄146 、 铂 、 苯 作用下, 140.0 ℃ 、1.33 Pa 条件下, 生成 5α-pregnan-3β-ol参考文献:名称:Facilitating Children's Understanding of Misinterpretation: Explanatory Efforts and Improvements in Perspective Taking摘要:The authors investigated children's understanding of how mistaken beliefs can arise through misinterpretation of ambiguous information. Children (N = 91), aged 4 to 5 years, were given pre- and posttests on their ability to infer a puppet's interpretation of a restricted-view drawing after the puppet had been led to an erroneous expectation about the drawing's identity. Before the posttest, the children received either self-explanation training or other-explanation training in which they explained the source of their own or a puppet's misinterpretations of drawings; a control group received no training. The children who received training improved from pre- to posttest, and those who had practiced explaining misinterpretations by referring to previously viewed pictures or to features of a target picture showed the greatest improvement. These results indicate that learning to explain misinterpretations can help children recognize situations in which misinterpretations are likely to occur.DOI:10.1080/00221320209598673
-
作为产物:参考文献:名称:Draczynska, Bozena; Tlomak, Elzbieta; Dmochowska-Gladysz, Jadwiga, Bulletin de l'Academie Polonaise des Sciences, Serie des Sciences Chimiques, 1982, vol. 30, # 1-12, p. 13 - 21摘要:DOI:
文献信息
-
[EN] IMIDAZOLIUM REAGENT FOR MASS SPECTROMETRY<br/>[FR] RÉACTIF D'IMIDAZOLIUM POUR SPECTROMÉTRIE DE MASSE申请人:HOFFMANN LA ROCHE公开号:WO2021234004A1公开(公告)日:2021-11-25The present invention relates to compounds which are suitable to be used in mass spectrometry as well as methods of mass spectrometric determination of analyte molecules using said compounds.本发明涉及适用于质谱的化合物,以及利用该化合物进行分析物分子的质谱测定方法。
-
Controllable Intramolecular Unactivated C(sp3)-H Amination and Oxygenation of Carbamates作者:Qihang Guo、Xiang Ren、Zhan LuDOI:10.1021/acs.orglett.8b03299日期:2019.2.15catalyst-controlled intramolecular unactivated C(sp3)-H amination and oxygenation of carbamates merging visible-light photocatalysis and earth-abundant transition metal catalysis have been reported. Useful amino alcohol and diol derivatives could be selectively obtained from readily available tertiary alcohol derivatives. The possible mechanisms have been proposed via a 1,5-HAT process followed by Lewis acid-controlled
-
Dithionite-Involved Multicomponent Coupling for Alkenyl and Alkyl Tertiary Sulfones作者:Yaping Li、Ming Wang、Xuefeng JiangDOI:10.1021/acs.orglett.1c01393日期:2021.6.18A dithionite-involved multicomponent reaction of redox-active esters and alkenes/alkynes is comprehensively achieved for the construction of alkyl and alkenyl tertiary sulfones. The industrial feedstock sodium dithionite is employed as a sulfur dioxide surrogate and a single-electron reductant to initiate the decarboxylation of redox-active esters. Mechanistic studies further indicated that the transformation
-
Steroid phosphate esters作者:Milon Sprecher、Ronald Breslow、Rachel Philosof-Oppenheimer、Eliram ChavetDOI:10.1016/s0040-4020(99)00193-3日期:1999.4used in the preparation and characterization of sundry steroid phosphate and phosphonate esters, as well as some P1,P2-disteroid pyrophosphates. An attempt to prepare cholest-3,5-dien-3-yl dialkyl phosphate by a vinylogous Perkow reaction from 6-bromocholest-4-en-3-one yielded only dienenones. As a model for P1,P2-disteroid pyrophosphate (and steroid phosphate) behavior in solution, the neutral crystalline
-
Pd-mediated cross-coupling of C-17 lithiated androst-16-en-3-ol – access to functionalized arylated steroid derivatives作者:Vanessa Koch、Stefan BräseDOI:10.1039/c6ob02496c日期:——we report on Pd-mediated cross-coupling of vinyllithium steroids and aryl bromides to introduce various substituted aryls at C-17 of steroidal frameworks based on the structure of epi-androsterone. Compared to other C–C cross-couplings, this method turned out to be an easy and competitive access to biologically interesting C-17 modified steroids.
表征谱图
-
氢谱1HNMR
-
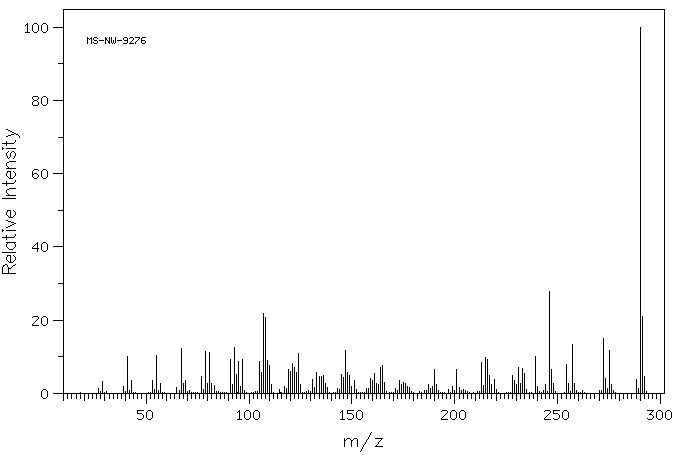
质谱MS
-
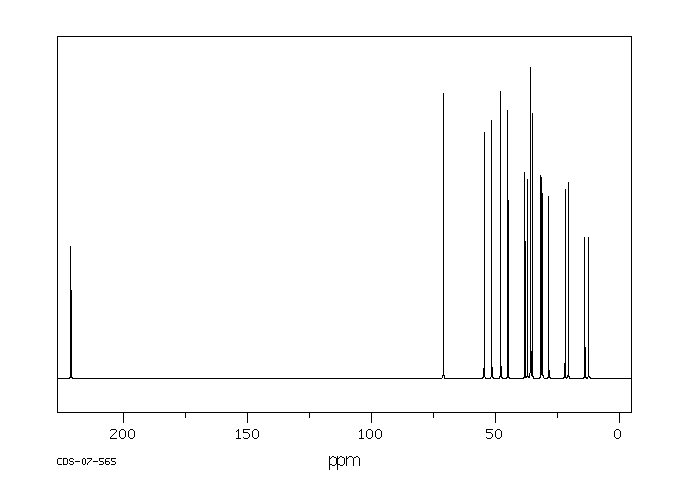
碳谱13CNMR
-
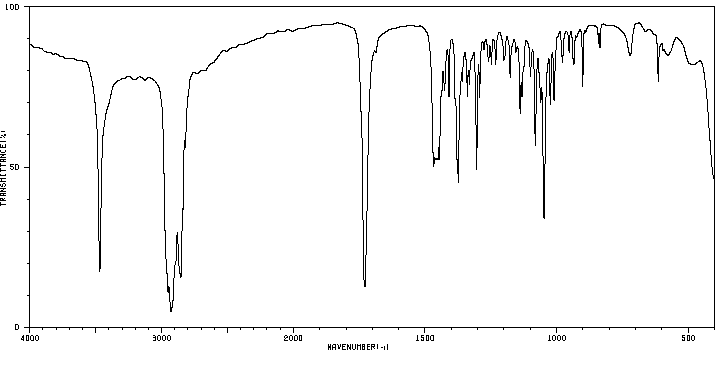
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B