泼尼松龙 | 50-24-8
物质功能分类
分子结构分类
中文名称
泼尼松龙
中文别名
孕甾-1,4-二烯-11β,17α,21-三醇-3,20-二酮;11β,17α,21-三羟基孕甾-1,4-二烯-3,20-二酮;11Beta,17Alpha,21-三羟基孕甾-1,4-二烯-3,20-二酮;去氢氢化可的松;氢化泼尼松;强的松龙
英文名称
prednisolon
英文别名
prednisolone;(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one
CAS
50-24-8
化学式
C21H28O5
mdl
MFCD00003649
分子量
360.45
InChiKey
OIGNJSKKLXVSLS-VWUMJDOOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:240 °C (dec.) (lit.)
-
比旋光度:D25 +102° (dioxane)
-
沸点:412.46°C (rough estimate)
-
密度:1.0963 (rough estimate)
-
闪点:2℃
-
溶解度:极微溶于水,溶于乙醇(96%)和甲醇,微溶于丙酮,微溶于二氯甲烷。
-
物理描述:Solid
-
颜色/状态:Crystals
-
气味:Odorless
-
蒸汽压力:1.18X10-13 mm Hg at 25 °C (est)
-
水溶性:-3.21
-
旋光度:Specific optical rotation: +102 deg at 25 °C/D (dioxane); max absorption (methanol): 242 nm (e= 15,000, a= 414, 1%, 1 cm)
-
碰撞截面:183.7 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:26
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:94.8
-
氢给体数:3
-
氢受体数:5
ADMET
代谢
泼尼松龙可以可逆地代谢为泼尼松,然后进一步代谢为17α,21-二羟基-孕甾-1,4,6-三烯-3,11,30-三酮(M-XVII),20α-二氢-泼尼松(M-V),6β-羟基-泼尼松(M-XII),6α-羟基-泼尼松(M-XIII)或20β-二氢-泼尼松(M-IV)。20β-二氢-泼尼松代谢为17α,20ξ,21-三羟基-5ξ-孕甾-1-烯-3,11-二酮(M-XVIII)。泼尼松龙代谢为Δ6-泼尼松龙(M-XI),20α-二氢-泼尼松龙(M-III),20β-二氢-泼尼松龙(M-II),6α-羟基-泼尼松龙(M-VII)或6β-羟基-泼尼松龙(M-VI)。6α-羟基-泼尼松龙代谢为6α,11β,17α,20β,21-五羟基-孕甾-1,4-二烯-3-酮(M-X)。6β-羟基-泼尼松龙代谢为6β,11β,17α,20β,21-五羟基-孕甾-1,4-二烯-3-酮(M-VIII),6β,11β,17α,20α,21-五羟基-孕甾-1,4-二烯-3-酮(M-IX)和6β,11β,17α,21-四羟基-5ξ-孕甾-1-烯-3,20-二酮(M-XIV)。M-VIII代谢为6β,11β,17α,20β,21-五羟基-5ξ-孕甾-1-烯-3-酮(M-XV)然后到M-XIV,而M-IX代谢为6β,11β,17α,20α,21-五羟基-5ξ-孕甾-1-烯-3-酮(M-XVI)然后到M-XIV。这些代谢物及其葡萄糖醛酸苷主要在尿液中排出。
Prednisolone can be reversibly metabolized to [prednisone] which is then metabolized to 17α,21-dihydroxy-pregnan-1,4,6-trien-3,11,30-trione (M-XVII), 20α-dihydro-prednisone (M-V), 6βhydroxy-prednisone (M-XII), 6α-hydroxy-prednisone (M-XIII), or 20β-dihydro-prednisone (M-IV). 20β-dihydro-prednisone is metabolized to 17α,20ξ,21-trihydroxy-5ξ-pregn-1-en-3,11-dione(M-XVIII). Prednisolone is metabolized to Δ6-prednisolone (M-XI), 20α-dihydro-prednisolone (M-III), 20β-dihydro-prednisolone (M-II), 6αhydroxy-prednisolone (M-VII), or 6βhydroxy-prednisolone(M-VI). 6αhydroxy-prednisolone is metabolized to 6α,11β,17α,20β,21-pentahydroxypregnan-1,4-diene-3-one (M-X). 6βhydroxy-prednisolone is metabolized to 6β,11β,17α,20β,21-pentahydroxypregnan-1,4-diene-3-one (M-VIII), 6β,11β,17α,20α,21-pentahydroxypregnan-1,4-diene-3-one (M-IX), and 6β,11β,17α,21-tetrahydroxy-5ξ-pregn-1-en-3,20-dione (M-XIV). MVIII is metabolized to 6β,11β,17α,20β,21-pentahydroxy-5ξ-pregn-1-en-3-one (M-XV) and then to MXIV, while MIX is metabolized to 6β,11β,17α,20α,21-pentahydroxy-5ξ-pregn-1-en-3-one (M-XVI) and then to MXIV. These metabolites and their glucuronide conjugates are excreted predominantly in the urine.
来源:DrugBank
代谢
4,5双键的还原可以在肝脏和肝脏外部位发生,产生一个无活性的物质。随后将3-酮基取代物还原为3-羟基以形成四氢可的松仅在肝脏中进行了演示。大多数环a-还原代谢物通过3-羟基与硫酸或葡萄糖醛酸酶促耦合,形成水溶性的硫酸酯或葡萄糖苷酸酯,并以这种方式被排出体外。
Reduction of the 4,5 double bond can occur at both hepatic and extrahepatic sites and yields an inactive substance. Subsequent reduction of the 3-ketone substituent to a 3-hydroxyl to form tetrahydrocortisol has been demonstrated only in liver. Most of the ring a - reduced metabolites are enzymatically coupled through the 3-hydroxyl with sulfate or with glucuronic acid to form water soluble sulfate esters or glucuronides, and they are excreted as such.
来源:Hazardous Substances Data Bank (HSDB)
代谢
主要在肝脏中结合,但也在肾脏中结合。/人类,口服/
Conjugated mostly in liver but also in kidney. /Human, oral/
来源:Hazardous Substances Data Bank (HSDB)
代谢
在当前研究中,使用基于组织培养介质199的灌注液,重新检查了在孤立、灌注、双循环的人胎盘小叶中泼尼松龙的代谢。通过将高效液相色谱(HPLC)和毛细管气相色谱(GC)测量的相对保留时间以及毛细管GC/MS记录的质量光谱与真实参考标准的进行比较,在6小时的灌注中,母体和胎儿室中都鉴定出了四种代谢物。类固醇作为MO-TMS醚进行衍生化以进行质谱测量。对五个灌注实验样本的分析得到了6小时灌注后的以下百分比转化(平均值+或-标准差,母体和胎儿灌注液,分别):泼尼松(49.1 +或- 7.8,49.1 +或- 6.6),20α-二氢泼尼松(0.84 +或- 0.29,0.81 +或- 0.35),20β-二氢泼尼松(39.1 +或- 6.7,39.2 +或- 5.9),20β-二氢泼尼松龙(6.8 +或- 2.7,6.3 +或- 1.6)和未代谢的泼尼松龙(4.1 +或- 1.8,4.6 +或- 2.1)。没有发现通过6β-羟基化或断裂C17-C20键形成的代谢物的证据。
In the present study the metabolism of prednisolone in the isolated, perfused, dual recirculating human placental lobule was reexamined, using a perfusate based on tissue culture medium 199. Four metabolites were identified in both the maternal and fetal compartments in 6 hr perfusions by comparison of relative retention times measured by HPLC and capillary GC and of mass spectra recorded by capillary GC/MS with those of authentic reference standards. The steroids were derivatized as the MO-TMS ethers for mass spectral measurements. Analysis of samples from five perfusion experiments resulted in the following percentage conversions after 6 hr perfusion (mean + or - standard deviation, maternal and fetal perfusate, respectively): prednisone (49.1 + or - 7.8, 49.1 + or - 6.6), 20 alpha-dihydroprednisone (0.84 + or - 0.29, 0.81 + or - 0.35), 20 beta-dihydroprednisone (39.1 + or - 6.7, 39.2 + or - 5.9), 20 beta-dihydroprednisolone (6.8 + or - 2.7, 6.3 + or - 1.6) and unmetabolized prednisolone (4.1 + or - 1.8, 4.6 + or - 2.1). No evidence was found for metabolites formed by 6 beta-hydroxylation or cleavage of the C17-C20 bond.
来源:Hazardous Substances Data Bank (HSDB)
代谢
进行了一项随机、四交叉研究,在八名健康男性志愿者中开展,旨在确定泼尼松(PN)和泼尼松龙(PL)的相对和绝对生物利用度。PN和PL分别以单次口服10毫克片剂和10毫克零级0.5小时静脉输注的方式给药。片剂治疗之间的PN和PL平均最大血浆浓度(Cmax)、达到Cmax的时间、血浆浓度-时间曲线下面积(AUC)和表观消除速率常数相似,表明PN和PL片剂生物等效。基于血浆PL浓度的绝对生物利用度(F)的确定与使用哪种静脉治疗作为参考无关,并表明从PN和PL片剂中PL的全身可用性是完全的。然而,基于血浆PN数据的F存在矛盾。以静脉PN为参考,从两种片剂中观察到大约70%的全身可用性,而以静脉PL为参考,全身可用性大于1。PN和PL是模型化合物,体现了在准确确定经历可逆代谢物质的相对和绝对生物利用度时涉及的困难。
A randomized, four-way cross-over study was conducted in eight healthy male volunteers to determine the relative and absolute bioavailability of prednisone (PN) and prednisolone (PL). PN and PL were administered as single, oral 10-mg tablet doses and as 10-mg zero-order 0.5-hour intravenous infusions. Comparable mean PN and PL maximum plasma concentrations (Cmax), times for Cmax, areas under the plasma concentration-time curves (AUC), and apparent elimination rate constants between tablet treatments demonstrated that PN and PL tablets were bioequivalent. Absolute bioavailability (F) determinations based on plasma PL concentrations were independent of which IV treatment was used as reference and indicated complete systemic availability of PL from both PN and PL tablets. However, F based on plasma PN data was contradictory. Using IV PN as reference, approximately 70% systemic availability was observed from both tablets, whereas using IV PL as reference, systemic availability was greater than unity. PN and PL are model compounds that exemplify the difficulties involved in accurately determining the relative and absolute bioavailability of substances that undergo reversible metabolism.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
DILI 注解:较少的药物性肝损伤关注
DILI Annotation:Less-DILI-Concern
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
严重性等级:3
Severity Grade:3
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
不良反应部分
Label Section:Adverse reactions
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
参考文献:M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. 用于研究药物诱导肝损伤的FDA批准药物标签,药物发现今日,16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank:按在人类中发展药物诱导肝损伤风险排名的最大参考药物清单。药物发现今日2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
References:M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
吸收、分配和排泄
Oral prednisolone reaches a Cmax of 113-1343ng/mL with a Tmax of 1.0-2.6 hours. Oral prednisolone is approximately 70% bioavailable.
来源:DrugBank
吸收、分配和排泄
0.15mg/kg剂量的泼尼松龙的分布容积为29.3L,而0.30mg/kg剂量的分布容积为44.2L。
A 0.15mg/kg dose of prednisolone has a volume of distribution of 29.3L, while a 0.30mg/kg dose has a volume of distribution of 44.2L.
来源:DrugBank
吸收、分配和排泄
0.15毫克/公斤剂量的泼尼松龙的清除率为0.09升/公斤/小时,而0.30毫克/公斤剂量的清除率为0.12升/公斤/小时。
A 0.15mg/kg dose of prednisolone has a clearance of 0.09L/kg/h, while a 0.30mg/kg dose has a clearance of 0.12L/kg/h.
来源:DrugBank
吸收、分配和排泄
进行了一项随机交叉研究,以比较8名患有慢性阻塞性肺疾病的患者(年龄63-81岁)和8名健康男性(年龄22-44岁)中,30毫克泼尼松龙普通口服片(Precortisyl)与肠溶片(Deltacortril)的药代动力学和药效学。尽管从肠溶片中药物吸收明显较慢,但两种制剂的血浆峰值水平和总浓度-时间曲线下面积是相当的。在志愿者中,肠溶片比普通片引起的肾上腺抑制显著减少。这种差异在患者中无统计学意义。在两组中,服用肠溶片后,血浆皮质醇水平的下降速度较慢。两组的血糖水平在8小时内均有所升高。结论是,在患有慢性阻塞性肺疾病的患者中,普通和肠溶泼尼松龙片的血浆峰值水平和总浓度-时间曲线下面积是相当的;肠溶片导致血浆皮质醇下降的滞后,在志愿者中,对皮质醇的抑制程度较轻。
A randomized crossover study was conducted to compare the pharmacokinetics and pharmacodynamics of 30 mg prednisolone in a plain oral tablet (Precortisyl) with those of an enteric coated tablet (Deltacortril) in 8 patients (ages 63-81 yr) with chronic obstructive pulmonary disease and in 8 healthy males (ages 22-44 yr). Although drug absorption was considerably slower from the enteric coated tablet, peak plasma levels and total area under the concn-time curve were equivalent for the formulations. Adrenal suppression was significantly less in volunteers after enteric coated than after plain tablets. This difference was not significant in patients. Plasma cortisol levels declined more slowly after enteric coated tablets in both groups. Blood glucose levels increased over 8 hr in both groups. It was concluded that in patients with chronic obstructive pulmonary disease, peak plasma levels and total area under the concn-time curve of plain and enteric coated prednisolone tablets are equivalent; enteric coated tablets result in a lag in the decline of plasma cortisol and, in volunteers, a less marked suppression of cortisol.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
安全说明:S16,S22,S26,S36/37/39,S45
-
危险品运输编号:NONH for all modes of transport
-
WGK Germany:3
-
海关编码:2937229000
-
危险品标志:Xn
-
危险类别码:R22
-
RTECS号:TU4152000
SDS
制备方法与用途
泼尼松龙又称氢化泼尼松、氯泼尼松、强的松龙、去氢氢化可的松,商品名为百力特。其化学名为醋酸泼尼松龙,是一种白色或几乎白色的结晶性粉末,无臭、味苦。几乎不溶于水,微溶于乙醇或氯仿。属肾上腺糖皮质激素及促皮质激素类药物,为中效糖皮质激素。其疗效与泼尼松相当,抗炎作用更强,对水盐代谢的影响较弱。
本品主要应用于羟基化合物(羟基位于11位),局部使用效果较好,如皮肤病和眼病等。因其在局部直接有效,也适用于肝功能不全患者。口服后吸收迅速且完全,约1小时达到血药浓度峰值,血浆蛋白结合率为90%~95%,半衰期约为200分钟。能进入胎儿循环,出现在乳汁中的药量为0.07%~0.23%。经肝内代谢,主要通过肾脏排泄,其中原形药物占20%以上。
泼尼松龙主要用于过敏性和炎症性疾病治疗。由于其潴钠作用较弱,通常不用于肾上腺皮质功能减退的替代疗法。对于肾上腺皮质机能亢进、高血压病、动脉粥样硬化、心力衰竭、糖尿病、精神病、癫痫、手术后患者以及胃、十二指肠溃疡、肠道疾病或慢性营养不良等病人应避免使用。一般感染性疾病不建议使用此类药物,但在急性感染中毒时必须与足量的有效抗菌药物联合应用;对于重度结核病,则需合并使用足量的抗结核药,并依据病情及时减量和停用。
不良反应与副作用
大量或长期使用本品可能导致肥胖、多毛症、痤疮、血糖升高、高血压、眼内压增高、钠水潴留、水肿、血钾降低、精神兴奋等症状。局部注射或外用时,有时会引起关节损伤、局部坏死、皮肤萎缩等问题。
化学性质
泼尼松龙为结晶化合物,熔点在225-233℃(240-241℃)之间。
用途
适用于类风湿关节炎、风湿热、红斑狼疮、皮肌炎、多发性骨髓瘤等疾病治疗,并可用于制取氢化泼尼松醋酸酯。用量根据关节大小而定,操作时需在无菌条件下进行,以防感染。
生产方法
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 醋酸泼尼松龙 prednisolone 21-acetate 52-21-1 C23H30O6 402.488 泼尼松龙戊酸酯 prednisolone 21-trimethylacetate 1107-99-9 C26H36O6 444.568 氢化可的松 HYDROCORTISONE 50-23-7 C21H30O5 362.466 泼尼松龙半琥珀酸酯 prednisolone hemisuccinate 2920-86-7 C25H32O8 460.524 泼尼松杂质D pregna-1,4,9(11)-triene-17α,21-diol-3,20-dione 10184-69-7 C21H26O4 342.435 泼尼松 Prednison 53-03-2 C21H26O5 358.434 双醋酸脱氢皮质醇 prednisolone 11β,21-diacetate 98523-85-4 C25H32O7 444.525 —— 21-<<(6-carboxy-3-cyclohexen-1-yl)carbonyl>oxy>-11β,17α-dihydroxypregna-1,4-diene-3,20-dione 3331-71-3 C29H36O8 512.6 醋酸氢化可的松 hydrocortisone acetate 50-03-3 C23H32O6 404.503 delta皮质素烯乙酸酯 pregna-1,4,9(11)-triene-17α,21-diol-3,20-dione 21-acetate 4380-55-6 C23H28O5 384.472 泼尼卡松 prednicarbate 73771-04-7 C27H36O8 488.578 —— [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] 3-(2-nitrophenyl)propanoate 1426535-37-6 C30H35NO8 537.61 —— [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] 3-(2-aminophenyl)propanoate 1154703-42-0 C30H39NO6 509.643 —— [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] 2-(2-nitrophenyl)acetate 1426535-35-4 C29H33NO8 523.583 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 迪普罗酮 11β,17α-dihydroxypregna-1,4-diene-3,20-dione 20423-99-8 C21H28O4 344.451 16alpha-羟基泼尼松龙 desonide 13951-70-7 C21H28O6 376.45 —— 21-fluoro-11β,17-dihydroxy-pregna-1,4-diene-3,20-dione 632-27-9 C21H27FO4 362.441 醋酸泼尼松龙 prednisolone 21-acetate 52-21-1 C23H30O6 402.488 泼尼松龙21-丙酸酯 11β,17-dihydroxy-21-propionyloxypregna-1,4-diene-3,20-dione 5740-62-5 C24H32O6 416.514 —— prednisolone 21-palmitate 54267-10-6 C37H58O6 598.864 —— Prednisolon-21-enanthat —— C28H40O6 472.622 泼尼松龙戊酸酯 prednisolone 21-trimethylacetate 1107-99-9 C26H36O6 444.568 氢化可的松 HYDROCORTISONE 50-23-7 C21H30O5 362.466 泼尼松龙半琥珀酸酯 prednisolone hemisuccinate 2920-86-7 C25H32O8 460.524 11beta,17,21-三羟基孕甾-1,4-二烯-3,20-二酮 21-(磷酸二氢酯) Prednisolone phosphate 302-25-0 C21H29O8P 440.43 —— 17,21-anhydroprednisolone 96168-88-6 C21H26O4 342.435 (11beta,17alpha)-11,17-二羟基-3-氧代雄甾-1,4-二烯-17-羧酸 Δ1-cortienic acid 37927-29-0 C20H26O5 346.423 (11B)-11,17-二羟基-21-[(甲基磺酰基)氧基]-孕甾-1,4-二烯-3,20-二酮 11β,17α,21-trihydroxypregna-1,4-diene-3,20-dione 21-mesylate 35410-28-7 C22H30O7S 438.542 17-脱羟基泼尼松龙 11β,21-Dihydroxy-pregn-1,4-diene-3,20-dione 13479-38-4 C21H28O4 344.451 司替泼尼松龙 prednisolone 21-Stearoylglycolate 5060-55-9 C41H64O8 684.954 泼尼松 Prednison 53-03-2 C21H26O5 358.434 双醋酸脱氢皮质醇 prednisolone 11β,21-diacetate 98523-85-4 C25H32O7 444.525 11,17,21-三羟基-3,20-二氧代孕甾烷-1,4-二烯-16-羧酸甲酯 P16CM 111802-47-2 C23H30O7 418.487 泼尼松龙17-醋酸酯 prednisolone Acetate 52628-64-5 C23H30O6 402.488 —— prednisolone 17,21-diacetate 17652-24-3 C25H32O7 444.525 瑞美松龙 rimexolone 49697-38-3 C24H34O3 370.532 —— 21-(N-tert-butyloxycarbonyl)aminoacetoxy-11β,17α-dihydroxypregna-1,4-diene-3,20-dione 135235-96-0 C28H39NO8 517.62 20-二氢泼尼松龙酸 11β,17α,20α-trihydroxy-3-oxo-1,4-pregnadien-21-oic acid 62358-12-7 C21H28O6 376.45 —— [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] 3-phenylpropanoate 95825-22-2 C30H36O6 492.612 20(R)-羟基泼尼松龙 (20R)-11β,17,20,21-tetrahydroxy-3-oxo-1,4-pregnadiene 15847-24-2 C21H30O5 362.466 20(S)-羟基强的松龙 11β,17α,20β,21-tetrahydroxypregna-1,4-dien-3-one 2299-46-9 C21H30O5 362.466 泼尼松龙 17-(碳酸乙酯)酯 Prednisolone 17-ethylcarbonate 104286-02-4 C24H32O7 432.514 —— ethyl 11β,17α-dihydroxyandrosta-1,4-dien-3-one-17β-carboxylate 182069-13-2 C22H30O5 374.477 21-二羟基-16&alpha-甲基孕甾-1,4,9(11)-三烯-3,20-二酮-21-醋酸酯 17α,21-dihydroxy-16α-methyl-1,4,9(11)-pregnatriene-3,20-dione 21-acetate 10106-41-9 C24H30O5 398.499 —— Prednisolon-21-benzoat —— C28H32O6 464.558 —— methylthiomethyl 11β,17α-dihydroxy-3-oxo-androst-1,4-diene-17β-carboxylate —— C22H30O5S 406.543 1,4-雄二烯-11-beta-醇-3,17-二酮 (8S,9S,10R,11S,13S,14S)-11-Hydroxy-10,13-dimethyl-7,8,9,10,11,12,13,14,15,16-decahydro-6H-cyclopenta[a]phenanthrene-3,17-dione 898-84-0 C19H24O3 300.398 醋酸泼尼松 prednisone acetate 125-10-0 C23H28O6 400.472 20-二氢泼尼松龙酸甲酯 methyl (20S)-11β,17,20-trihydroxy-3-oxo-1,4-pregnadien-21-oate 57073-10-6 C22H30O6 390.477 泼尼卡松 prednicarbate 73771-04-7 C27H36O8 488.578 —— (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-17-[2-(4-methylpyrimidin-2-yl)sulfanylacetyl]-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one 1168141-37-4 C26H32N2O4S 468.617 —— prednisolone-17α,21-ethyl orthopropanoate 61774-32-1 C26H36O6 444.568 脱氧强的松龙-16-烯 17a-dehydroxyprednisolone 3103-17-1 C21H26O4 342.435 21-O-乙酰基地塞米松9,11-环氧化物 17α,21-dihydroxy-9β,11β-epoxy-16α-methylpregna-1,4-diene-3,20-dione 21-acetate 2884-51-7 C24H30O6 414.499 —— 11β-(trimethylsiloxy)-16α,17α,21-trimethylpregna-1,4-dien-3,20-dione 220983-31-3 C27H42O3Si 442.714 11beta, 17,21-三羟基孕甾-1,4-二烯-3,20-二酮 17-苯甲酸酯 17α-benzoyloxy-11β,21-dihydroxy-1,4-pregnadiene-3,20-dione 31311-74-7 C28H32O6 464.558 —— 11β-hydroxy-1,4,16-trienepregna-3,20-dione 60124-50-7 C21H26O3 326.436 —— Prednisolone 17α,21-methyl orthopentanoate 39791-30-5 C27H38O6 458.595 NO-泼尼松龙 prednisolone 21-[(4’-nitro-oxymethyl)benzoate] 327610-87-7 C29H33NO9 539.582 —— ethyl 17α-formyloxy-11β-hydroxyandrosta-1,4-dien-3-one-17β-carboxylate —— C23H30O6 402.488 泼尼莫司汀 Prednimustine 29069-24-7 C35H45Cl2NO6 646.651 —— 11β,17α-dihydroxy-3,20-dioxopregna-1,4-dien-21-yl β-D-cellobioside 92901-27-4 C33H48O15 684.735 —— (20S)-21-(methylamino)-11β,17,20-trihydroxy-3,21-dioxo-1,4-pregnadiene 110417-82-8 C22H31NO5 389.492 —— (20R)-21-(methylamino)-11β,17,20-trihydroxy-3,21-dioxo-1,4-pregnadiene 110417-81-7 C22H31NO5 389.492 —— 17α-ethoxycarbonyloxy-11β-hydroxy-3-oxoandrosta-1,4-diene-17β-carboxylic acid —— C23H30O7 418.487 —— (20S)-21-(ethylamino)-11β,17,20-trihydroxy-3,21-dioxo-1,4-pregnadiene 110417-84-0 C23H33NO5 403.519 —— (20R)-21-(ethylamino)-11β,17,20-trihydroxy-3,21-dioxo-1,4-pregnadiene 110417-83-9 C23H33NO5 403.519 L-丙氨酸,N-[(二甲氨基)亚甲基]-,[N(E)]- (20S)-21-(n-propylamino)-11β,17,20-trihydroxy-3,21-dioxo-1,4-pregnadiene 97142-21-7 C24H35NO5 417.546 —— (20R)-21-(n-propylamino)-11β,17,20-trihydroxy-3,21-dioxo-1,4-pregnadiene 97142-22-8 C24H35NO5 417.546 —— 21-(N-tert-butyloxycarbonyl)-(γ-tert-butyl ester)-α-L-glutamyloxy-11β-17α-dihydroxypregna-1,4-diene-3,20-dione 135260-60-5 C35H51NO10 645.791 [2-[(8S,9S,10R,11S,13S,14S)-11-羟基-10,13-二甲基-3-氧代-6,7,8,9,11,12,14,15-八氢环戊烯并[a]菲-17-基]-2-氧代乙基]乙酸酯 17a-prednisolone dehydroxyacetate 3044-42-6 C23H28O5 384.472 —— [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] 3-(2-nitrophenyl)propanoate 1426535-37-6 C30H35NO8 537.61 —— 11β-(trimethylsiloxy)-pregna-1,4,16-trien-3,20-dione 60124-51-8 C24H34O3Si 398.618 —— 21-methyl-11β-(trimethylsiloxy)-pregna-1,4,16-trien-3,20-dione 207346-24-5 C25H36O3Si 412.645 —— [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] 2-(2-nitrophenyl)acetate 1426535-35-4 C29H33NO8 523.583 (8S,9S,10R,11S,13S,14S)-17-(2,2-二氯乙酰基)氧基-11-羟基-10,13-二甲基-3-氧代-7,8,9,11,12,14,15,16-八氢-6H-环戊[a]菲-17-羧酸乙酯 Etiprednol 199331-40-3 C24H30Cl2O6 485.405 依碳氯替泼诺 Loteprednol etabonate 82034-46-6 C24H31ClO7 466.959 —— 21-Acetoxy-3,20-dioxo-16-methyl-Δ1,4,9(11)-pregnatrien 4258-83-7 C24H30O4 382.5 —— (20S)-21-(benzylamino)-11β,17,20-trihydroxy-3,21-dioxo-1,4-pregnadiene 104008-75-5 C28H35NO5 465.59 —— (20R)-21-(benzylamino)-11β,17,20-trihydroxy-3,21-dioxo-1,4-pregnadiene 104008-74-4 C28H35NO5 465.59 1-去氢肾上腺甾酮 androsta-1,4-diene-3,11,17-trione 7738-93-4 C19H22O3 298.382 —— 9β,11β-epoxy-21-hydroxypregna-1,4,16-triene-3,20-dione 21-acetate 103466-44-0 C23H26O5 382.456 —— (8S,9S,10R,11S,13S,14S,17R)-17-(2-(benzo[d]thiazol-2-ylthio)acetyl)-11,17-dihydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one 1168140-52-0 C28H31NO4S2 509.69 —— 16α,21-dimethyl-11β,20-bis(trimethylsiloxy)-pregna-1,4,17(20)-trien-3-one 226712-41-0 C29H48O3Si2 500.869 —— 21-hydroxypregna-1,4,9(11),16-tetraene-3,20-dione —— C21H24O3 324.42 21-羟基孕甾-1,4,9(11),16-四烯-3,20-二酮-21-醋酸酯 tetraene acetate 37413-91-5 C23H26O4 366.457 —— [(8S,9S,10R,11S,13S,14S,17R)-11-hydroxy-10,13-dimethyl-17-[2-(4-methylpyrimidin-2-yl)sulfanylacetyl]-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl] furan-2-carboxylate 1345277-94-2 C31H34N2O6S 562.687 —— [(8S,9S,10R,11S,13S,14S,17R)-11-hydroxy-17-(2-isoquinolin-1-ylsulfanylacetyl)-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl] furan-2-carboxylate 1345277-91-9 C35H35NO6S 597.732 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
反应信息
-
作为反应物:描述:泼尼松龙 在 sodium periodate 、 水 、 potassium carbonate 、 potassium hydrogencarbonate 作用下, 以 四氢呋喃 、 甲醇 、 二氯甲烷 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 6.42h, 生成 (8S,9S,10R,11S,13S,14S)-17-(2,2-二氯乙酰基)氧基-11-羟基-10,13-二甲基-3-氧代-7,8,9,11,12,14,15,16-八氢-6H-环戊[a]菲-17-羧酸乙酯参考文献:名称:[EN] POTENT SOFT ANTI-INFLAMMATORY CORTICOSTEROID COMPOUNDS AND USES THEREOF
[FR] COMPOSÉS CORTICOSTÉROÏDES ANTI-INFLAMMATOIRES DOUX PUISSANTS ET LEURS UTILISATIONS摘要:公开号:WO2018232007A8 -
作为产物:参考文献:名称:Application of urethane prepolymers to immobilization of biocatalysts: .DELTA.1-dehydrogenation of hydrocortisone by Arthrobacter simplex cells entrapped with urethane prepolymers.摘要:通过将细胞悬浮液与由甲苯二异氰酸酯和聚醚二醇合成的水溶性聚氨酯预聚物混合,固定了具有显著类固醇Δ1-脱氢酶活性的丙酮干燥的单纯节杆菌细胞。在氢化可的松转化为泼尼松龙的过程中,被固定的细胞活性受聚氨酯预聚物特性的影响,如预聚物中异氰酸酯基团的含量、聚醚二元醇的分子量和二元醇中环氧乙烷的含量。在水性反应混合物中加入 10% 的有机溶剂,如甲醇和乙二醇,可大大提高底物的溶解度和固定化细胞的反应速率。即使在含有 30% 甲醇的体系中,固定化细胞的活性仍然很高,而游离细胞的活性则受到严重抑制。电子受体(酚嗪甲硫酸盐或 2,6-二氯苯酚靛酚)的存在极大地刺激了固着细胞和游离细胞的类固醇转化。固定化技术大大提高了细胞在重复反应中的稳定性。DOI:10.1271/bbb1961.44.1119
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
DISUBSTITUTED TRIFLUOROMETHYL PYRIMIDINONES AND THEIR USE申请人:BAYER PHARMA AKTIENGESELLSCHAFT公开号:US20160221965A1公开(公告)日:2016-08-04The present application relates to novel 2,5-disubstituted 6-(trifluoromethyl)pyrimidin-4(3H)-one derivatives, to processes for their preparation, to their use alone or in combinations for the treatment and/or prevention of diseases, and to their use for preparing medicaments for the treatment and/or prevention of diseases, in particular for treatment and/or prevention of cardiovascular, renal, inflammatory and fibrotic diseases.
-
[EN] 2-QUINOLONE DERIVED INHIBITORS OF BCL6<br/>[FR] INHIBITEURS DE BCL6 DÉRIVÉS DE 2-QUINOLONE申请人:CANCER RESEARCH TECH LTD公开号:WO2018215798A1公开(公告)日:2018-11-29The present invention relates to compounds of formula I that function as inhibitors of BCL6(B- cell lymphoma 6) activity: Formula I wherein X1, X2, X3, R1, R2, R3, R4 and R5 are each as defined herein. The present invention also relates to processes for the preparation of these compounds, to pharmaceutical compositions comprising them, and to their use in the treatment of proliferative disorders, such as cancer,as well as other diseases or conditions in which BCL6 activity is implicated.本发明涉及作为BCL6(B细胞淋巴瘤6)活性抑制剂的I式化合物:式中X1、X2、X3、R1、R2、R3、R4和R5分别如本文所定义。本发明还涉及制备这些化合物的方法,包括含有它们的药物组合物,以及它们在治疗增生性疾病(如癌症)以及其他BCL6活性所涉及的疾病或病况中的用途。
-
[EN] INHIBITORS OF BRUTON'S TYROSINE KINASE<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON申请人:BIOCAD JOINT STOCK CO公开号:WO2018092047A1公开(公告)日:2018-05-24The present invention relates to a new compound of formula I: or pharmaceutically acceptable salt, solvate or stereoisomer thereof, wherein: V1 is C or N, V2 is C(R2) or N, whereby if V1 is C then V2 is N, if V1 is C then V2 is C(R2), or if V1 is N then V2 is C(R2); each n, k is independently 0, 1; each R2, R11 is independently H, D, Hal, CN, NR'R", C(O)NR'R", C1-C6 alkoxy; R3 is H, D, hydroxy, C(O)C1-C6 alkyl, C(O)C2-C6 alkenyl, C(O)C2-C6 alkynyl, C1-C6 alkyl; R4 is H, Hal, CN, CONR'R", hydroxy, C1-C6 alkyl, C1-C6 alkoxy; L is CH2, NH, O or chemical bond; R1 is selected from the group of the fragments, comprising: Fragment 1, Fragment 2, Fragment 3 each A1, A2, A3, A4 is independently CH, N, CHal; each A5, A6, A7, A8, A9 is independently C, CH or N; R5 is H, CN, Hal, CONR'R", C1-C6 alkyl, non-substituted or substituted by one or more halogens; each R' and R" is independently selected from the group, comprising H, C1-C6 alkyl, C1-C6 cycloalkyl, aryl; R6 is selected from the group: [formula II] each R7, R8, R9, R10 is independently vinyl, methylacetylenyl; Hal is CI, Br, I, F, which have properties of inhibitor of Bruton's tyrosine kinase (Btk), to pharmaceutical compositions containing such compounds, and their use as pharmaceuticals for treatment of diseases and disorder.本发明涉及一种新的化合物,其化学式为I:或其药学上可接受的盐、溶剂化合物或立体异构体,其中:V1为C或N,V2为C(R2)或N,如果V1为C,则V2为N,如果V1为C,则V2为C(R2),或者如果V1为N,则V2为C(R2);每个n,k独立地为0或1;每个R2,R11独立地为H,D,Hal,CN,NR'R",C(O)NR'R",C1-C6烷氧基;R3为H,D,羟基,C(O)C1-C6烷基,C(O)C2-C6烯基,C(O)C2-C6炔基,C1-C6烷基;R4为H,Hal,CN,CONR'R",羟基,C1-C6烷基,C1-C6烷氧基;L为CH2,NH,O或化学键;R1从包括的片段组中选择:片段1,片段2,片段3,每个A1,A2,A3,A4独立地为CH,N,CHal;每个A5,A6,A7,A8,A9独立地为C,CH或N;R5为H,CN,Hal,CONR'R",C1-C6烷基,未取代或被一个或多个卤素取代;每个R'和R"独立地从包括H,C1-C6烷基,C1-C6环烷基,芳基的组中选择;R6从组中选择:[化学式II]每个R7,R8,R9,R10独立地为乙烯基,甲基乙炔基;Hal为CI,Br,I,F,具有布鲁顿酪氨酸激酶(Btk)抑制剂的性质,以及含有这种化合物的药物组合物,以及它们作为治疗疾病和紊乱的药物的用途。
-
[EN] DIHYDROPYRROLONAPHTYRIDINONE COMPOUNDS AS INHIBITORS OF JAK<br/>[FR] COMPOSÉS DE DIHYDROPYRROLONAPHTYRIDINONE COMME INHIBITEURS DE JAK申请人:TAKEDA PHARMACEUTICAL公开号:WO2010144486A1公开(公告)日:2010-12-16Disclosed are JAK inhibitors of formula (I) where G1, R1, R2, R3, R4, R5, R6, and R7 are defined in the specification. Also disclosed are pharmaceutical compositions, kits and articles of manufacture which contain the compounds, methods and materials for making the compounds, and methods of using the compounds to treat diseases, disorders, and conditions involving the immune system and inflammation, including rheumatoid arthritis, hematological malignancies, epithelial cancers (i.e., carcinomas), and other diseases, disorders or conditions associated with JAK.揭示了式(I)的JAK抑制剂,其中G1、R1、R2、R3、R4、R5、R6和R7在规范中定义。还披露了含有这些化合物的药物组合物、试剂盒和制造物品,制备这些化合物的方法和材料,以及使用这些化合物治疗涉及免疫系统和炎症的疾病、紊乱和症状的方法,包括类风湿关节炎、血液恶性肿瘤、上皮癌(即癌症)和其他与JAK相关的疾病、紊乱或症状。
表征谱图
-
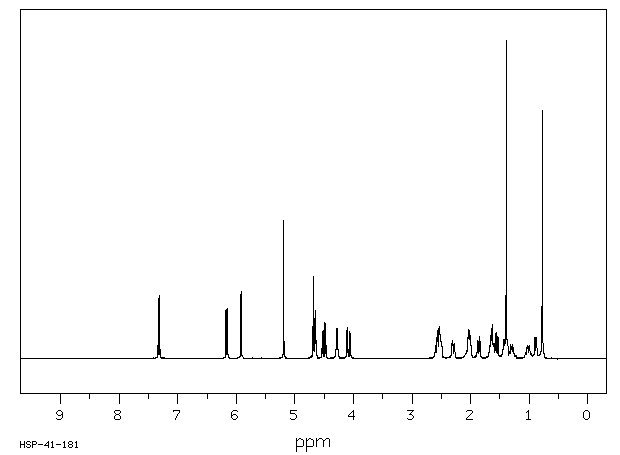
氢谱1HNMR
-
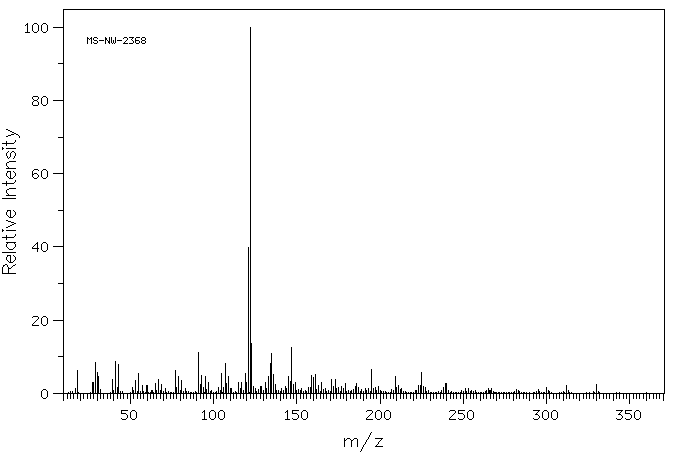
质谱MS
-
碳谱13CNMR
-
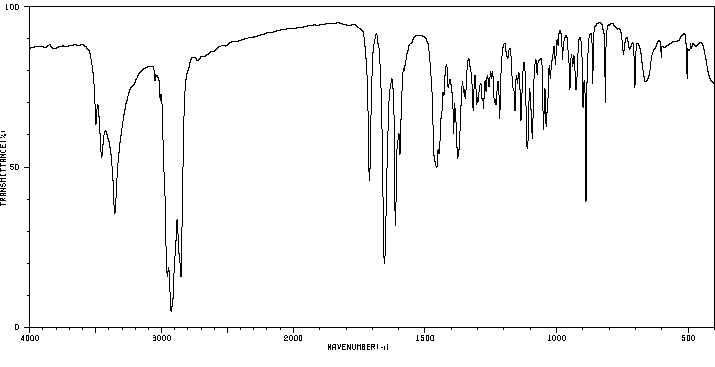
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B