氢化可的松 | 50-23-7
物质功能分类
分子结构分类
中文名称
氢化可的松
中文别名
氢化皮质素;11β,17α,21-三羟基孕甾-4-烯-3,20-二酮;皮质醇;17-羥皮甾酮;可的索;可的唑;11Beta,17Alpha,21-三羟基孕甾-4-烯-3,20-二酮
英文名称
HYDROCORTISONE
英文别名
cortisol;11β,17α,21-trihydroxypregn-4-ene-3,20-dione;(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one
CAS
50-23-7
化学式
C21H30O5
mdl
MFCD00011654
分子量
362.466
InChiKey
JYGXADMDTFJGBT-VWUMJDOOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:211-214 °C(lit.)
-
比旋光度:166 º (c=1, C2H5OH 25 ºC)
-
沸点:414.06°C (rough estimate)
-
密度:1.0812 (rough estimate)
-
闪点:220°C
-
溶解度:H2O:100 mg/mL
-
LogP:1.610
-
物理描述:Solid
-
颜色/状态:Plates from alc or iso-propyl alc
-
气味:Odorless
-
味道:Bitter taste
-
蒸汽压力:1.35X10-13 mm Hg at 25 °C (est)
-
水溶性:-2.97
-
稳定性/保质期:
SENSITIVE TO LIGHT
-
旋光度:Specific optical rotation: +167 deg at 22 °C/D (absolute alc)
-
Caco2细胞的药物渗透性:-4.66
-
碰撞截面:189.1 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:26
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.809
-
拓扑面积:94.8
-
氢给体数:3
-
氢受体数:5
ADMET
代谢
氢化可的松通过CYP3A代谢成6-β-氢化可的松,通过3-氧代-5-β-类固醇4-脱氢酶代谢成5-β-四氢可的松,通过3-氧代-5-α-类固醇4-脱氢酶2代谢成5-α-四氢可的松,通过皮质类固醇11-β-脱氢酶同酶1和皮质类固醇11-β-脱氢酶同酶2代谢成可的松,以及代谢成葡萄糖苷酸产物。可的松进一步代谢成四氢可的松和二氢可的松。
Hydrocortisone is metabolised to 6-beta hydrocortisol via CYP3A, 5-beta tetrahydrocortisol via 3-oxo-5-beta-steroid 4-dehydrogenase, 5-alpha tetrahydrocortisol via 3-oxo-5-alpha-steroid 4-dehydrogenase 2, cortisone via Corticosteroid 11-beta-dehydrogenase isozyme 1 and Corticosteroid 11-beta-dehydrogenase isozyme 2, and glucuronide products. Cortisone is further metabolized to tetrahydrocortisone and dihydrocortisol.
来源:DrugBank
代谢
对完整和照射的大鼠在循环灌注条件下,对外源性氢化可的松的吸收及其代谢物在孤立肝脏中的形成进行了研究。结果显示,照射大鼠肝脏对激素的吸收大大降低,但在照射肝脏灌注介质中发现的多数代谢物的含量与对照相比有所增加。这表明照射抑制了氢化可的松代谢产物后续的转化。
A study was made of the absorption of exogenous hydrocortisone and formation of its metabolites in isolated liver of intact and exposed rats in conditions of recirculating perfusion. It was shown that the absorption of the hormone by the liver of irradiated rats was greatly lowered but the content of most metabolites found in the perfused medium of irradiated liver increased as compared to the control. It is suggested that irradiation inhibits subsequent transformations of the hydrocortisone metabolism products.
来源:Hazardous Substances Data Bank (HSDB)
代谢
(3)H-氢化可的松及其代谢物在完整和链脲佐菌素诱导的糖尿病大鼠的肝脏和肾脏的亚细胞分布进行了研究。给药这种激素后10分钟,在肝脏胞浆、微粒体、线粒体和细胞核中发现了几种代谢物(主要是四氢可的松)和原激素,各种亚细胞组分中个别化合物的相对含量不同。在链脲佐菌素诱导的糖尿病大鼠的肝脏线粒体、微粒体和细胞核中,四氢可的松的浓度降低,而原激素的浓度增加,与正常动物相比。研究表明,在糖尿病动物中发现的这种变化可能是转录和翻译过程对糖皮质激素敏感性增加的一个原因。在完整大鼠的肾脏胞浆和微粒体中发现了可的松和四氢可的松。然而,在糖尿病动物中,四氢可的松的浓度增加,而可的松的浓度无法检测到。
Subcellular distribution of (3)H-hydrocortisone and its metabolites in the liver and kidney of intact and alloxan diabetic rats was investigated. Ten minutes after the administration of this hormone several metabolites (mostly tetrahydrocortisol) and the native hormone were found in liver cytosol, microsomes, mitochondria and nuclei, the relative content of individual compounds in various subcellular fractions being different. In liver mitochondria, microsomes and nuclei of alloxan diabetic rats the concentration of tetrahydrocortisol was decreased, while that of native hormone was increased as compared to normal animals. It was suggested that such changes found in diabetic animals may be one of the causes of increased sensitivity of transcription and translation processes to glucocorticoids. In kidney cytosol and microsomes of intact rats cortisone and tetrahydrocortisol were found. In diabetic animals, however, the concentration of tetrahydrocortisol increased, while that of cortisone was undetectable.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
DILI 注解:无 DILI(药物性肝损伤)担忧
DILI Annotation:No-DILI-Concern
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
标签部分:没有匹配项
Label Section:No match
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
参考文献:M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. 用于研究药物诱导肝损伤的FDA批准药物标签,药物发现今天,16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank:按在人类中发展药物诱导肝损伤风险排名的最大参考药物清单。药物发现今天 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
References:M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
◉ 母乳喂养期间使用概述:氢化可的松(皮质醇)是母乳的正常组成部分,但在药理剂量外源性给药后,对乳汁中的氢化可的松进行研究。尽管氢化可的松达到危险量的可能性不大,但可能更倾向于使用研究更为充分的替代皮质类固醇。母亲使用氢化可的松灌肠不太可能在母乳喂养的婴儿中引起任何不良反应。局部母亲注射,例如用于肌腱炎,也不太可能在母乳喂养的婴儿中引起任何不良反应,但偶尔可能导致暂时性的乳汁供应减少。参见氢化可的松,外用。
母乳中的皮质醇可能在肠道成熟、肠道微生物群、生长、身体成分或神经发育中发挥作用,但缺乏充分的研究。浓度遵循昼夜节律,早上约7:00时浓度最高,下午和晚上浓度最低。母亲的压力可以增加母乳中皮质醇的水平。母乳中的皮质醇可能有助于预防婴儿后期的肥胖,尤其是女孩;然而,在另一项研究中,乳汁糖皮质激素水平与1岁时体脂百分比、肥胖度和头围呈正相关。
母乳中的皮质醇浓度不受在室温下储存36小时、多次冷冻和解冻循环的影响,Holder巴氏杀菌(62.5°C,30分钟)对其影响也非常小。
◉ 对母乳喂养婴儿的影响:未见任何系统性皮质类固醇有报道。
◉ 对泌乳和母乳的影响:截至修订日期,未找到关于氢化可的松对血清催乳素或哺乳母亲泌乳影响的信息。然而,有报道称,将中到大剂量的储库型皮质类固醇注射到关节中会导致暂时性的泌乳减少。
一项对46名在34周前分娩妇女的研究发现,另一种皮质类固醇(倍他米松,两次肌肉注射,每次11.4 mg,间隔24小时)在分娩前3至9天内给药,会导致延迟乳生成II期,并在分娩后10天内平均乳汁量减少。如果婴儿在母亲接受皮质类固醇后少于3天或多于10天分娩,乳汁量不会受到影响。等效剂量的氢化可的松可能有相同的效果。
一项对87名孕妇的研究发现,孕期给予上述剂量的倍他米松会导致孕期过早刺激乳糖分泌。尽管增加在统计学上显著,但临床意义似乎很小。等效剂量的氢化可的松可能有相同的效果。
◉ Summary of Use during Lactation:Hydrocortisone (cortisol) is a normal component of breastmilk, but it has not been studied in milk after exogenous administration in pharmacologic amounts. Although it is unlikely that dangerous amounts of hydrocortisone would reach the infant, a better studied alternate corticosteroid might be preferred. Maternal use of hydrocortisone as an enema would not be expected to cause any adverse effects in breastfed infants. Local maternal injections, such as for tendinitis, would not be expected to cause any adverse effects in breastfed infants, but might occasionally cause temporary loss of milk supply. See also Hydrocortisone, Topical.
Cortisol in breastmilk might have a role in intestinal maturation, the intestinal microbiome, growth, body composition or neurodevelopment, but adequate studies are lacking. Concentrations follow a diurnal rhythm, with the highest concentrations in the morning at about 7:00 am and the lowest concentrations in the late afternoon and evening. Maternal stress can increase breastmilk cortisol levels. Cortisol in milk may protect against later infant obesity, especially in girls; however, in another study, milk glucocorticoid levels were positively associated with percent fat mass, adiposity and head circumference at 1 year of age.
Cortisol concentrations in breastmilk are not affected by storage for 36 hours at room temperature, during multiple freeze-thaw cycles, and very little by Holder pasteurization (62.5 degrees C for 30 minutes).
◉ Effects in Breastfed Infants:None reported with any systemic corticosteroid.
◉ Effects on Lactation and Breastmilk:Published information on the effects of hydrocortisone on serum prolactin or on lactation in nursing mothers was not found as of the revision date. However, medium to large doses of depot corticosteroids injected into joints have been reported to cause temporary reduction of lactation.
A study of 46 women who delivered an infant before 34 weeks of gestation found that a course of another corticosteroid (betamethasone, 2 intramuscular injections of 11.4 mg of betamethasone 24 hours apart) given between 3 and 9 days before delivery resulted in delayed lactogenesis II and lower average milk volumes during the 10 days after delivery. Milk volume was not affected if the infant was delivered less than 3 days or more than 10 days after the mother received the corticosteroid. An equivalent dosage regimen of hydrocortisone might have the same effect.
A study of 87 pregnant women found that betamethasone given as above during pregnancy caused a premature stimulation of lactose secretion during pregnancy. Although the increase was statistically significant, the clinical importance appears to be minimal. An equivalent dosage regimen of hydrocortisone might have the same effect.
来源:Drugs and Lactation Database (LactMed)
吸收、分配和排泄
口服氢化可的松的剂量为0.2-0.3毫克/千克/天时,平均Cmax达到32.69nmol/L,平均AUC为90.63h*nmol/L。0.4-0.6毫克/千克/天的剂量使平均Cmax达到70.81nmol/L,平均AUC为199.11h*nmol/L。然而,氢化可的松的药代动力学在患者之间可能相差10倍。局部氢化可的松乳膏的生物利用度为4-19%[8546995],Tmax为24小时。氢化可的松保留灌肠的生物利用度在慢吸收者为0.810,在快吸收者为0.502。慢吸收者吸收氢化可的松的速度为0.361±0.255/小时,快吸收者的吸收速度为1.05±0.255/小时。20毫克静脉注射剂量的氢化可的松的AUC为1163±277ng*h/mL。
Oral hydrocortisone at a dose of 0.2-0.3mg/kg/day reached a mean Cmax of 32.69nmol/L with a mean AUC of 90.63h\*nmol/L A 0.4-0.6mg/kg/day dose reached a mean Cmax of 70.81nmol/L with a mean AUC of 199.11h\*nmol/L. However, the pharmacokinetics of hydrocortisone can vary by 10 times from patient to patient. Topical hydrocortisone cream is 4-19% bioavailable[8546995] with a Tmax of 24h. Hydrocortisone retention enemas are have a bioavailability of 0.810 for slow absorbers and 0.502 in rapid absorbers. Slow absorbers take up hydrocortisone at a rate of 0.361±0.255/h while fast absorbers take up hydrocortisone at a rate of 1.05±0.255/h. A 20mg IV dose of hydrocortisone has an AUC of 1163±277ng\*h/mL.
来源:DrugBank
吸收、分配和排泄
皮质类固醇主要通过尿液排出。然而,关于确切比例的数据并不容易获得。
Corticosteroids are eliminated predominantly in the urine. However, data regarding the exact proportion is not readily available.
来源:DrugBank
吸收、分配和排泄
总氢化可的松的分布容积为39.82升,而游离部分的分布容积为474.38升。
Total hydrocortisone has a volume of distribution of 39.82L, while the free fraction has a volume of distribution of 474.38L.
来源:DrugBank
吸收、分配和排泄
Total hydrocortisone by the oral route has a mean clearance of 12.85L/h, while the free fraction has a mean clearance of 235.78L/h. A 20mg IV dose of hydrocortisone has a clearance of 18.2±4.2L/h.
来源:DrugBank
吸收、分配和排泄
Following percutaneous penetration of a topical corticosteroid, the drug that is systemically absorbed probably follows the metabolic pathways of systemically administered corticosteroids. Corticosteroids usually are metabolized in the liver and excreted by the kidneys. Some topical corticosteroids and their metabolites are excreted in bile. /Topical corticosteroids/
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S36/37,S45
-
危险类别码:R62,R63
-
WGK Germany:3
-
海关编码:2937210000
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:GM8925000
SDS
制备方法与用途
氢化可的松简介
简介
氢化可的松(Hydrocortisone,简称HC)也称皮质醇或可的索(Cortisol),是一种重要的肾上腺糖皮质激素。其化学名称为11β,17α,21-三羟基孕甾-4-烯-3,20-二酮。氢化可的松通过弥散作用于靶细胞,与其受体相结合形成类固醇-受体复合物。激活后的类固醇-受体复合物作为基因转录的激活因子以二聚体的形式与DNA上的特异性顺序链结合,调控基因转录并增加mRNA生成。最终在靶标细胞内合成相应的蛋白质,实现类固醇激素的生理和药理效应。氢化可的松具有抗炎、抗病毒、抗休克及抗过敏等作用。
应用应用
主要应用于肾上腺皮质功能减退症的替代治疗以及先天性肾上腺皮质功能增生症的治疗,同时也用于类风湿性关节炎、风湿性发热、痛风、支气管哮喘和过敏性疾病。此外,HC还可用于严重感染及抗休克治疗等。HC还作为制备其他几种重要甾体药物的原料药。
适应症适应症
氢化可的松主要用于肾上腺皮质功能减退症的替代治疗以及先天性肾上腺皮质功能增生症的治疗,同时也适用于类风湿性关节炎、风湿性发热、痛风、支气管哮喘和过敏性疾病。此外,HC还用于严重感染及抗休克治疗等。
药理作用药理作用
氢化可的松的主要药理作用包括:
- 抗炎作用:通过降低机体毛细血管通透性、稳定溶酶体膜活性和防止白细胞在炎症部位堆积,减轻或预防炎症反应。
- 免疫抑制作用:加速机体内淋巴细胞凋亡,阻止单核巨噬细胞的形成,从而防止或抑制细胞介导的免疫反应。
- 抗病毒作用:对抗细菌内毒素对机体的损害,发挥抗病毒的作用。氢化可的松与地塞米松类似,但不良反应较轻。
制备
甾体药物半合成法的起始原料是甾醇衍生物,如从穿地龙、黄姜等植物根茎中萃取的薯芋皂素,或从剑麻中萃取的剑麻皂苷。梨头霉(Absidia orchidis 3.65)能直接在化合物S上引入β-羟基,从而缩短合成氢化可的松的工艺路线。
化学性质
- 外观:白色或类白色结晶性粉末
- 气味与味道:无臭、微苦
- 遇光变质
- 熔点(Mp):214-220℃(分解)
- 旋光度:[α]25D +157.5°(二噁烷)、+176°(氯仿)、+162°-+169°(乙醇)
- 吸收峰:在242nm波长处有最大吸收
- 溶解性:不溶于水,难溶于乙醚,微溶于氯仿,可溶于丙酮、乙醇,并能与浓硫酸液反应呈绿色荧光
LD50(大鼠,腹腔)150mg/kg
用途
主要用于生化研究和肾上腺皮质激素类药物。
糖皮质激素具有抗炎、抗过敏、抗毒素及抗休克四大作用。
生产方法
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 11-表氢化可的松 Epicortisol 566-35-8 C21H30O5 362.466 —— 18-Hydroxycortisol 86002-90-6 C21H30O6 378.5 脱氧可的松 Cortexolone 152-58-9 C21H30O4 346.467 醋酸氢化可的松 hydrocortisone acetate 50-03-3 C23H32O6 404.503 泼尼松龙 prednisolon 50-24-8 C21H28O5 360.45 —— 3,11β,17α,21-tetrahydroxypregn-4-en-20-one —— C21H32O5 364.482 11beta,17,21-三羟基孕甾-4-烯-3,20-二酮 21-特戊酸酯 Hydrocortisone pivalate 24869-41-8 C26H38O6 446.584 氢化可的松琥珀酸酯 hydrocortisone hemisuccinate 2203-97-6 C25H34O8 462.54 —— hydrocortisone-succinate-hydrocortisone —— C46H62O12 806.991 17Alpha-羟基黄体酮 17-hydroxyprogesterone 68-96-2 C21H30O3 330.467 可的松 Cortisone 53-06-5 C21H28O5 360.45 孕甾-4-烯-17Alpha,21-二醇-3,20-二酮-21-醋酸酯 cortexolone 21-acetate 640-87-9 C23H32O5 388.504 丁酸氢化可的松 hydrocortisone 17α-butyrate 13609-67-1 C25H36O6 432.557 醋酸可的松 Cortisone acetate 50-04-4 C23H30O6 402.488 17,21-二羟基孕甾-4-烯-3,20-二酮17-乙酸酯 17α,21-dihydroxy-4-pregnene-3,20-dione 17-acetate 19357-45-0 C23H32O5 388.504 醋酸阿奈可他 anecortave 7753-60-8 C23H30O5 386.488 地塞米松 dexamethasone 23495-06-9 C22H29FO5 392.468 甘珀酸 carbenoxolone 5697-56-3 C34H50O7 570.767 黄体酮 Progesterone 57-83-0 C21H30O2 314.468 5beta-二氢皮质醇21-乙酸酯 5β-dihydrocortisol 21-acetate 64313-94-6 C23H34O6 406.519 孕烯醇酮 Pregnenolone 145-13-1 C21H32O2 316.484 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 11-beta,17-alpha,21-三羟基-6-alpha-甲基孕甾-4-烯-3,20-二酮 11β,17α,21-trihydroxy-6α-methylpregna-4-ene-3,20-dione 1625-39-4 C22H32O5 376.493 11-表氢化可的松 Epicortisol 566-35-8 C21H30O5 362.466 11beta,17alpha-二羟基-4-孕烯-3,20-二酮 21-deoxycortisol 641-77-0 C21H30O4 346.467 (6S,8S,9S,10R,11S,13S,14S,17R)-17-乙酰基-11,17-二羟基-6,10,13-三甲基-2,6,7,8,9,11,12,14,15,16-十氢-1H-环戊并[a]菲-3-酮 11β,17-dihydroxy-6α-methyl-pregn-4-ene-3,20-dione 7055-53-0 C22H32O4 360.494 21-去氢皮质醇 11β,17α-dihydroxy-3,20-dioxopregn-4-en-21-al 14760-49-7 C21H28O5 360.45 氢化可的松杂质D 6β,11β,17α,21‐tetrahydroxypregen‐4‐ene‐3,20‐dione 53-35-0 C21H30O6 378.466 脱氧可的松 Cortexolone 152-58-9 C21H30O4 346.467 醋酸氢化可的松 hydrocortisone acetate 50-03-3 C23H32O6 404.503 氢化可的松 21-丁酸盐 hydrocortisone 21-butyrate 6677-99-2 C25H36O6 432.557 氢化可的松21-戊酸酯 hydrocortisone 17-valerate 6678-00-8 C26H38O6 446.584 4-孕烯-11beta,17alpha,21-三醇-3,20-二酮 21-辛酸盐 21-octanoyloxy-11β,17α-dihydroxypregn-4-en-3,20-dione 6678-14-4 C29H44O6 488.665 [2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-二羟基-10,13-二甲基-3-氧代-2,6,7,8,9,11,12,14,15,16-十氢-1H-环戊二烯并[a]菲-17-基]-2-氧代乙基]己酸酯 21-hexanoyloxy-11β,17α-dihydroxypregn-4-en-3,20-dione 3593-96-2 C27H40O6 460.611 —— 21-octadecanoyloxy-11β,17α-dihydroxypregn-4-en-3,20-dione 81047-65-6 C39H64O6 628.934 环戊丙酸氢化可的松 hydrocortisone cypionate 508-99-6 C29H42O6 486.649 11beta,17,21-三羟基孕甾-4-烯-3,20-二酮 21-(3,3-二甲基丁酸酯) 21-cortisol tert-butylacetate 508-96-3 C27H40O6 460.611 6beta-甲泼尼龙 6β-methylprednisolone 18462-27-6 C22H30O5 374.477 泼尼松龙 prednisolon 50-24-8 C21H28O5 360.45 氢化可的松琥珀酸酯 hydrocortisone hemisuccinate 2203-97-6 C25H34O8 462.54 氢化可的松体杂质20 21-(3-Carboxy-1-oxopropoxy)-17α-hydroxy-11α-hydroxypregna-4-ene-3,20-dione 103242-89-3 C25H34O8 462.54 —— hydrocortisone-succinate-hydrocortisone —— C46H62O12 806.991 (8S,9S,10R,11S,13S,14S)-11,17-二羟基-10,13-二甲基-3-氧代-2,6,7,8,9,11,12,14,15,16-十氢-1H-环戊二烯并[a]菲-17-羧酸 cortienic acid 3597-45-3 C20H28O5 348.439 皮质醇21-硫酸盐 4-pregnene-11β,17α,21-triol-3,20-dione 21-sulfate 1253-43-6 C21H30O8S 442.53 —— (8S,9S,10R,11S,13S,14S,17R)-11-hydroxy-10,13-dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dodecahydrospiro[cyclopenta[a]phenanthrene-17,2′-oxetane]-3,3′(2H)-dione 77826-00-7 C21H28O4 344.451 氢化可的松体杂质21 9α,11β,17α,21-tetrahydroxypregn-4-ene-3,20-dione 58809-70-4 C21H30O6 378.466 11beta,17,21-三羟基孕甾-4-烯-3,20-二酮 21-甲烷磺酸酯 Cortisol 21-mesylate 6677-96-9 C22H32O7S 440.558 可的松 Cortisone 53-06-5 C21H28O5 360.45 肾上腺酮 Corticosterone 50-22-6 C21H30O4 346.467 —— corticosterone 50-22-6 C21H30O4 346.467 11beta,17,21-三羟基孕甾-4-烯-3,20-二酮 17-乙酸酯 hydrocortisone 17-acetate 16463-74-4 C23H32O6 404.503 氢化可的松17-丙酸酯 hydrocortisone 17-propionate 65980-97-4 C24H34O6 418.53 丁酸氢化可的松 hydrocortisone 17α-butyrate 13609-67-1 C25H36O6 432.557 (11beta,20S)-11,17,20,21-四羟基孕甾-4-烯-3-酮 11β,17α,20R,21-tetrahydroxypregn-4-en-3-one 116-58-5 C21H32O5 364.482 20-aLpha-二氢可的松 11β,17α,20β,21-tetrahydroxypregn-4-en-3-one 1719-79-5 C21H32O5 364.482 —— Reichstein's substance E —— C21H32O5 364.5 11beta-羟基睾酮 11β-hydroxytestosterone 1816-85-9 C19H28O3 304.43 醋丙氢可的松 Hydrocortisone aceponate 74050-20-7 C26H36O7 460.568 氢化可的松11,17,21-三乙酸酯 11β,17α,21-triacetoxy-pregn-4-ene-3,20-dione 3517-51-9 C27H36O8 488.578 21-乙酸肾上腺酮 corticosterone-21-acetate 1173-26-8 C23H32O5 388.504 —— methylthiomethyl 11β,17α-dihydroxy-3-oxo-androst-4-ene-17β-carboxylate 84099-87-6 C22H32O5S 408.559 醋酸可的松 Cortisone acetate 50-04-4 C23H30O6 402.488 4-雄烯-11β-醇-3,17-二酮 (8S,9S,10R,11S,13S,14S)-11-Hydroxy-10,13-dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dodecahydro-2H-cyclopenta[a]phenanthrene-3,17-dione 382-44-5 C19H26O3 302.414 —— cortisol 3-(O-carboxymethyl)oxime 109527-48-2 C23H33NO7 435.518 —— 4-(2-carboxyethylthio)cortisol 74997-23-2 C24H34O7S 466.596 11 -酮睾酮 11-ketotestosterone 564-35-2 C19H26O3 302.414 11-氧代睾酮 17-hydroxyandrost-4-ene-3,11-dione 53187-98-7 C19H26O3 302.414 11beta,17,21-三羟基孕甾-4-烯-3,20-二酮 17-苯甲酸酯 7-(benzoyloxy)-11,21-dihydroxy-(11β)-pregn-4-ene-3,20-dione 28956-89-0 C28H34O6 466.574 —— 11β, 17-Dihydroxy-21-[(3-pyridinylcarbonyl)oxy]pregn-4-ene-3,20-dione —— C27H33NO6 467.562 —— 6β,11β-dihydroxy-4-androstene-3,17-dione 54668-66-5 C19H26O4 318.413 20(R)-羟基泼尼松龙 (20R)-11β,17,20,21-tetrahydroxy-3-oxo-1,4-pregnadiene 15847-24-2 C21H30O5 362.466 氧戊环 tetrahydrocortisol 53-02-1 C21H34O5 366.498 异体-3Α-四氢皮质醇 3α,11β,17α,21-tetrahydroxy-5α-pregnan-20-one 302-91-0 C21H34O5 366.498 —— 3β,11β,17,21-tetrahydroxy-5β-pregnan-20-one 15734-50-6 C21H34O5 366.498 5α-孕烷-3β,11β,17α,21-四醇-20-酮 5α-pregnane-3β,11β,17α,21-tetraol-20-one 651-43-4 C21H34O5 366.498 11-Beta,17-alpha,21-三羟基-5-beta-孕烯-3,20-二酮 5β-pregnane-11β,17α,21-triol-3,20-dione 1482-50-4 C21H32O5 364.482 11-Beta,17-alpha,21-三羟基-5-alpha-孕烯-3,20-二酮 11β,17α,21-trihydroxy-5α-pregnane-3,20-dione 516-41-6 C21H32O5 364.482 —— dihydrocortisol 16355-27-4 C21H32O5 364.482 [2-[(8S,9S,10R,11R,13S,14S,17R)-11,17-二羟基-10,13-二甲基-3-氧代-2,6,7,8,9,11,12,14,15,16-十氢-1H-环戊并[a]菲-17-基]-2-氧代-乙基](2S,3S,4S,5R)-2,3,4,5-四羟基-6-氧代-己酸酯 cortisol 21-glucuronide 7301-54-4 C27H38O11 538.592 9-去氢睾酮 17β-Hydroxy-androsta-4,9(11)-dien-3-on 2398-99-4 C19H26O2 286.414 —— chloromethyl 11β-hydroxy-17α-methoxycarbonyloxy-3-oxo-androst-4-ene-17β-carboxylate —— C23H31ClO7 454.948 —— (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-17-(2-quinoxalin-2-ylsulfanylacetyl)-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one 1166866-65-4 C29H34N2O4S 506.666 —— (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-17-(2-quinolin-2-ylsulfanylacetyl)-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one 1166866-58-5 C30H35NO4S 505.678 —— (8S,9S,10R,11S,13S,14S,17R)-17-[2-(1,3-benzoxazol-2-ylsulfanyl)acetyl]-11,17-dihydroxy-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one 1168140-44-0 C28H33NO5S 495.64 肾上腺甾酮 adrenosterone 382-45-6 C19H24O3 300.398 —— 11β-hydroxy-17α,20:20,21-bis(methylenedioxy)pregn-4-en-3-one 807-05-6 C23H32O6 404.503 —— (20Ξ)-11β-methoxymethoxy-17,20;20,21-bis-methylenedioxy-pregn-4-en-3-one 107035-16-5 C25H36O7 448.557 (8S,9S,10R,11S,13S,14S)-17-(2,2-二氯乙酰基)氧基-11-羟基-10,13-二甲基-3-氧代-7,8,9,11,12,14,15,16-八氢-6H-环戊[a]菲-17-羧酸乙酯 Etiprednol 199331-40-3 C24H30Cl2O6 485.405 —— (8S,9S,10R,11S,13S,14S,17R)-17-[2-(1,3-benzothiazol-2-ylsulfanyl)acetyl]-11,17-dihydroxy-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one 1166869-57-3 C28H33NO4S2 511.706 —— 4-androstene-3,6,11,17-tetraone 79037-18-6 C19H22O4 314.381 —— (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-17-[2-([1,3]thiazolo[4,5-b]pyridin-2-ylsulfanyl)acetyl]-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one 1166866-59-6 C27H32N2O4S2 512.694 —— [(8S,9S,10R,11S,13S,14S,17R)-11-hydroxy-10,13-dimethyl-3-oxo-17-(2-pyrimidin-2-ylsulfanylacetyl)-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl] furan-2-carboxylate 1166866-43-8 C30H34N2O6S 550.676 雄甾-4,9(11)-二烯-3,17-二酮 androst-4,9(11)-dien-3,17-dione 1035-69-4 C19H24O2 284.398 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
反应信息
-
作为反应物:参考文献:名称:Novel hydrocortisone derivative摘要:本发明的17-α-丁酸酯-11-β-羟基-21-丙酸酯-4-孕烯-3,20-二酮,即本发明的羟考的松17-丁酸酯21-丙酸酯是通过将羟考的松17-丁酸酯与丙酸酐或卤化物酰化而制备的。本发明的化合物具有优异的抗炎作用、经皮吸收性能和较少的副作用。公开号:US04290962A1
-
作为产物:参考文献:名称:一种从丁酸氢化可的松母液中回收氢化可的 松的方法摘要:一种从丁酸氢化可的松母液中回收氢化可的松的方法,包括以下步骤:将丁酸氢化可的松精制母液加入甲醇浓缩至粘稠,然后加入混合溶剂搅拌溶清,降温。在惰性气体的保护下滴加碱液,加毕保温反应。经中和、浓缩、冷冻过滤、烘干得到氢化可的松。本发明通过采用惰性气体保护和混合溶剂相结合的反应方式,有效避免因甾核D环结构断裂所致化合物分解而无法回收物料的情况。本发明方法具有操作简便,处理时间短,生产成本低,生产安全性和物料稳定性高,对环境不会造成污染;回收率高,回收得到的氢化可的松纯度高,干燥后可以直接作为原料生产丁酸氢化可的松。公开号:CN104356187B
-
作为试剂:参考文献:名称:铂单晶表面催化的异丁烷,正己烷和正庚烷的氘交换反应摘要:DOI:10.1021/j100232a019
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] DIHYDROPYRROLONAPHTYRIDINONE COMPOUNDS AS INHIBITORS OF JAK<br/>[FR] COMPOSÉS DE DIHYDROPYRROLONAPHTYRIDINONE COMME INHIBITEURS DE JAK申请人:TAKEDA PHARMACEUTICAL公开号:WO2010144486A1公开(公告)日:2010-12-16Disclosed are JAK inhibitors of formula (I) where G1, R1, R2, R3, R4, R5, R6, and R7 are defined in the specification. Also disclosed are pharmaceutical compositions, kits and articles of manufacture which contain the compounds, methods and materials for making the compounds, and methods of using the compounds to treat diseases, disorders, and conditions involving the immune system and inflammation, including rheumatoid arthritis, hematological malignancies, epithelial cancers (i.e., carcinomas), and other diseases, disorders or conditions associated with JAK.揭示了式(I)的JAK抑制剂,其中G1、R1、R2、R3、R4、R5、R6和R7在规范中定义。还披露了含有这些化合物的药物组合物、试剂盒和制造物品,制备这些化合物的方法和材料,以及使用这些化合物治疗涉及免疫系统和炎症的疾病、紊乱和症状的方法,包括类风湿关节炎、血液恶性肿瘤、上皮癌(即癌症)和其他与JAK相关的疾病、紊乱或症状。
-
[EN] NOVEL COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS THEREOF FOR THE TREATMENT OF INFLAMMATORY DISORDERS<br/>[FR] NOUVEAUX COMPOSÉS ET COMPOSITIONS PHARMACEUTIQUES LES COMPRENANT POUR LE TRAITEMENT DE TROUBLES INFLAMMATOIRES申请人:GALAPAGOS NV公开号:WO2017012647A1公开(公告)日:2017-01-26The present invention discloses compounds according to Formula (I), wherein R1, R3, R4, R5, L1, and Cy are as defined herein. The present invention also provides compounds, methods for the production of said compounds of the invention, pharmaceutical compositions comprising the same and their use in allergic or inflammatory conditions, autoimmune diseases, proliferative diseases, transplantation rejection, diseases involving impairment of cartilage turnover, congenital cartilage malformations, and/or diseases associated with hypersecretion of IL6 and/or interferons. The present invention also methods for the prevention and/or treatment of the aforementioned diseases by administering a compound of the invention.本发明公开了根据式(I)的化合物,其中R1、R3、R4、R5、L1和Cy如本文所定义。本发明还提供了该发明的化合物、制备该化合物的方法、包括相同化合物的药物组合物以及它们在过敏或炎症症状、自身免疫疾病、增殖性疾病、移植排斥、涉及软骨周转障碍的疾病、先天软骨畸形和/或与IL6和/或干扰素过度分泌相关的疾病中的使用。本发明还提供了通过给予该发明的化合物来预防和/或治疗上述疾病的方法。
-
[EN] ARYL ETHER-BASE KINASE INHIBITORS<br/>[FR] INHIBITEURS DE KINASES DE TYPE ARYLÉTHER-BASE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2015038112A1公开(公告)日:2015-03-19The present disclosure is generally directed to compounds which can inhibit AAK1 (adaptor associated kinase 1), compositions comprising such compounds, and methods for inhibiting AAK1.
表征谱图
-
氢谱1HNMR
-
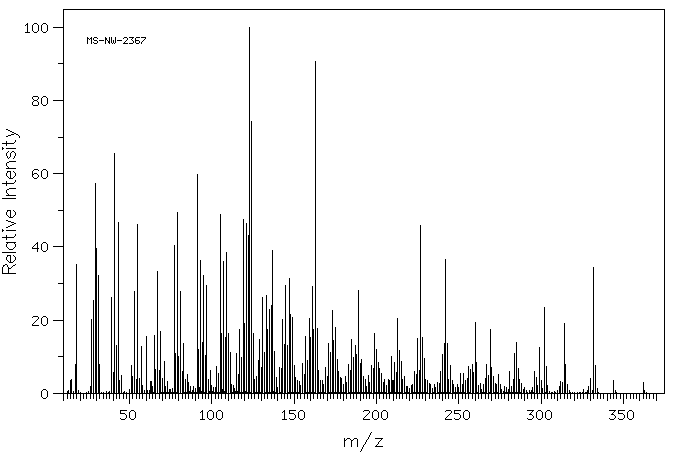
质谱MS
-
碳谱13CNMR
-
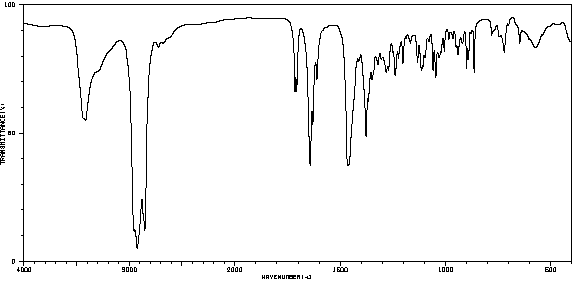
红外IR
-
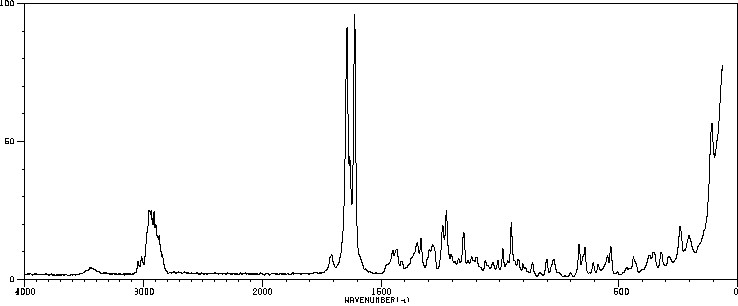
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B