代谢
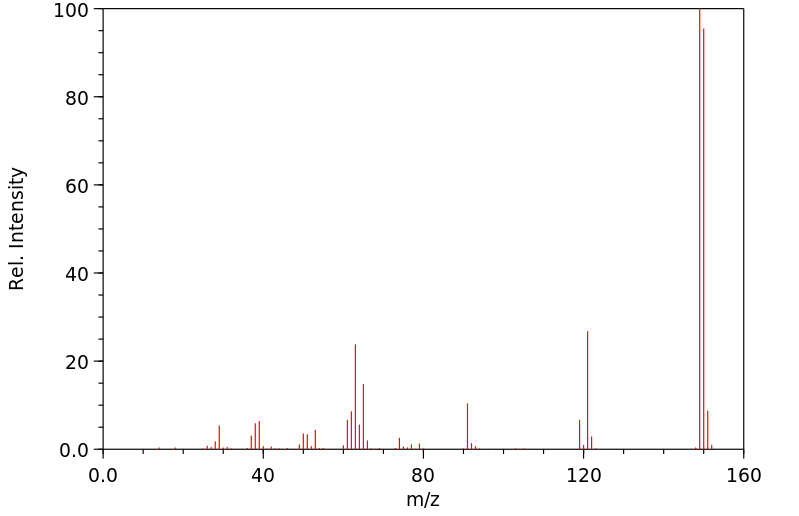
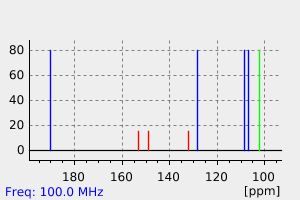
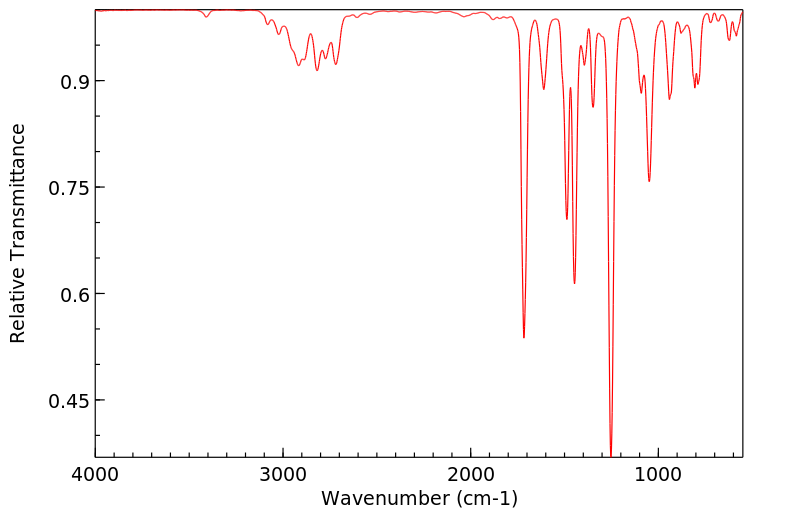
Paths of metabolism: piperonal converted to piperonyl alcohol which is metabolized to glucoside conjugate. /From table/
来源:Hazardous Substances Data Bank (HSDB)