甲基 1,3-苯并二恶唑-5-羧酸酯 | 326-56-7
中文名称
甲基 1,3-苯并二恶唑-5-羧酸酯
中文别名
1,3-苯并二氧代-5-羧酸甲酯;甲基1,3-苯并二恶唑-5-羧酸酯
英文名称
methyl 3,4-methylenedioxybenzoate
英文别名
methyl benzo[d][1,3]dioxole-5-carboxylate;methyl piperonylate;methyl benzo[1,3]dioxole-5-carboxylate;methyl 1,3-benzodioxole-5-carboxylate
CAS
326-56-7
化学式
C9H8O4
mdl
MFCD00030273
分子量
180.16
InChiKey
QCHGUEIECOASJU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:50°C
-
沸点:272.96°C (rough estimate)
-
密度:1.2933 (rough estimate)
-
LogP:1.376 (est)
-
保留指数:1932
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:13
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
储存条件:2-8℃,干燥,密封保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Methyl 1,3-benzodioxole-5-carboxylate
Synonyms: Methyl 3,4-methylenedioxybenzoate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 1,3-benzodioxole-5-carboxylate
CAS number: 326-56-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H8O4
Molecular weight: 180.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Methyl 1,3-benzodioxole-5-carboxylate
Synonyms: Methyl 3,4-methylenedioxybenzoate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 1,3-benzodioxole-5-carboxylate
CAS number: 326-56-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H8O4
Molecular weight: 180.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 胡椒酸 Piperonylic acid 94-53-1 C8H6O4 166.133 胡椒醇 piperonol 495-76-1 C8H8O3 152.15 胡椒醛 piperonal 120-57-0 C8H6O3 150.134 甲基-6-溴苯并[d][1,3]二氧杂环戊烯-5-羧酸甲酯 methyl 6-bromo-1,3-benzodioxole-5-carboxylate 61441-09-6 C9H7BrO4 259.056 3,4-二羟基苯甲酸甲酯 3,4-dihydroxybenzoic acid methyl ester 2150-43-8 C8H8O4 168.149 —— methyl 6-iodobenzo[d][1,3]dioxole-5-carboxylate 61599-80-2 C9H7IO4 306.057 1-(3,4-亚甲二氧基苯基)乙醇 1-benzo[1,3]dioxol-5-yl-ethanol 6329-73-3 C9H10O3 166.177 胡椒酸酰氯 3,4-(methylenedioxy)benzoyl chloride 25054-53-9 C8H5ClO3 184.579 原儿茶酸 3,4-Dihydroxybenzoic acid 99-50-3 C7H6O4 154.122 —— 6-iodopiperonal 58343-53-6 C8H5IO3 276.03 (6-碘苯并[1,3]二氧杂环戊烯-5-基)甲醇 6-iodo-1,3-benzodioxole-5-methanol 69048-76-6 C8H7IO3 278.046 —— 1,6-bis(3,4-methylenedioxyphenyl)hexane-1,6-diol 214334-35-7 C20H22O6 358.391 —— 1,6-bis(3,4-methylenedioxyphenyl)hexane-1,6-dione 6268-56-0 C20H18O6 354.359 黄樟素 1-allyl-3,4-methylenedioxybenzene 94-59-7 C10H10O2 162.188 (Z)-5-(丙烯-1-基)-1,3-苯并二氧戊环 (Z)-5-(prop-1-en-1-yl)benzo[d][1,3]dioxole 17627-76-8 C10H10O2 162.188 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 胡椒酸 Piperonylic acid 94-53-1 C8H6O4 166.133 —— isopropyl benzo[d][1,3]dioxole-5-carboxylate 95633-79-7 C11H12O4 208.214 —— methyl 6-hydroxybenzo[d][1,3]dioxole-5-carboxylate 82146-04-1 C9H8O5 196.16 —— phenyl benzo[d][1,3]dioxole-5-carboxylate 32745-63-4 C14H10O4 242.231 胡椒醇 piperonol 495-76-1 C8H8O3 152.15 胡椒醛 piperonal 120-57-0 C8H6O3 150.134 3,4-二羟基苯甲酸甲酯 3,4-dihydroxybenzoic acid methyl ester 2150-43-8 C8H8O4 168.149 —— methyl 6-aminobenzo[d][1,3]dioxole-5-carboxylate 40680-63-5 C9H9NO4 195.175 6-氨基-1,3-苯并二氧代-5-羧酸 4,5-methylenedioxyanthranilic acid 20332-16-5 C8H7NO4 181.148 —— (R)-2-<3',4'-(methylenedioxy)phenyl>oxirane 133789-65-8 C9H8O3 164.161 胡椒酸酰氯 3,4-(methylenedioxy)benzoyl chloride 25054-53-9 C8H5ClO3 184.579 1,3-苯并间二氧杂环戊烯-5-羧酰胺 3,4-methylenedioxybenzamide 4847-94-3 C8H7NO3 165.148 —— methyl (4-hydroxy-2,3,5,6-tetrachlorophenoxy)-1,3-benzodioxole-5-carboxylate —— C15H8Cl4O6 426.037 3,4-(亚甲二氧基)甲苯 5-methyl-1,3-benzodioxole 7145-99-5 C8H8O2 136.15 3,4-亚甲基二氧苄肼 benzo[d][1,3]dioxole-5-carbohydrazide 22026-39-7 C8H8N2O3 180.163 —— 2-(3,4-methylenedioxyphenyl)ethanonyl methyl sulfide 77263-37-7 C10H10O3S 210.254 3-(1,3-苯并二氧代-5-基)-3-氧代丙腈 3-benzo[1,3]dioxol-5-yl-3-oxo-propionitrile 96220-14-3 C10H7NO3 189.17 —— 1-(benzo[1,3]dioxol-5-yl)pent-4-en-1-one 114908-30-4 C12H12O3 204.225 —— piperonoylazide 54022-34-3 C8H5N3O3 191.146 —— N-(2-aminoethyl)-1,3-benzdioxol-5-carboxamide 94319-95-6 C10H12N2O3 208.217 —— 3',4'-(methylenedioxy)-2-(methylsulfinyl)acetophenone 56221-32-0 C10H10O4S 226.253 1-苯并[1,3]二氧代-5-丁烷-1,3-二酮 1-(benzo[d][1,3]dioxol-5-yl)butane-1,3-dione 56221-42-2 C11H10O4 206.198 —— benzo[1,3]dioxol-5-yl-diphenyl-methanol 265983-88-8 C20H16O3 304.345 —— 1-(3,4-methylenedioxybenzoyl)thiosemicarbazide 52190-69-9 C9H9N3O3S 239.255 3-(6-胡椒环基)-3-氧代丙酸乙酯 ethyl 3-(benzo[d][1,3]dioxol-5-yl)-3-oxopropanoate 81581-27-3 C12H12O5 236.224 —— 3,4-dimethoxy-(1'-hydroxy-1'-methylethyl)benzene 74385-18-5 C11H16O3 196.246 5-[(E)-丙-1-烯基]苯并[1,3]二氧杂环戊烯 isosafrole 4043-71-4 C10H10O2 162.188 (Z)-5-(丙烯-1-基)-1,3-苯并二氧戊环 (Z)-5-(prop-1-en-1-yl)benzo[d][1,3]dioxole 17627-76-8 C10H10O2 162.188 6-硝基-1,3-苯并二氧戊环-5-羧酸甲酯 methyl 6-nitrobenzo[d][1,3]dioxole-5-carboxylate 721-00-6 C9H7NO6 225.158 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:128.一种将酸转化为醛的新方法摘要:DOI:10.1039/jr9360000584
-
作为产物:描述:参考文献:名称:分子简化策略设计的新型二茂铁-N-酰基腙的合成、结构表征和抗菌活性庆祝Paulo Freire教授诞辰100周年摘要:追求新的具有生物活性和结构多样化的分子是药物化学的主要核心,这可以通过不同的理论和实验策略来实现,包括分子简化。从更复杂的结构模型开始,可以设计出具有更容易合成要求、改进的物理化学性质甚至新的生物学特征的新分子。基于该策略,已规划、合成并筛选了36 种二茂铁基-N-酰基腙 (Fc-NAH) 杂化物的抗菌活性。应用的合成方法导致了E的立体选择性-异构体,避免可能影响生物学研究的非对映体混合物。与起始结构模型相反,Fc-NAH 衍生物显示出显着的抗微生物活性。化合物SintMed(75-110)对革兰氏阳性菌株B. subtilis和S. aureus尤其有效,并且由于其最佳结果必须突出显示硝基取代的衍生物。事实上,衍生物SintMed77(3,5-二硝基苯基)和SintMed93(4-硝基苯基)是该系列中最活跃的,抑制区分别为 20.1 ± 0.52 和 21.4 ± 0.83。可以通过体外DOI:10.1016/j.jorganchem.2022.122488
文献信息
-
Palladium-Catalyzed Visible-Light-Driven Carboxylation of Aryl and Alkenyl Triflates by Using Photoredox Catalysts作者:Katsuya Shimomaki、Tomoya Nakajima、Joaquim Caner、Naoyuki Toriumi、Nobuharu IwasawaDOI:10.1021/acs.orglett.9b01340日期:2019.6.21A visible-light-driven carboxylation of aryl and alkenyl triflates with CO2 is developed by using a combination of Pd and photoredox catalysts. This reaction proceeds under mild conditions and can be applied to a wide range of substrates including acyclic alkenyl triflates.
-
[EN] ACTIVATORS OF THE RETINOIC ACID INDUCIBLE GENE "RIG-I' PATHWAY AND METHODS OF USE THEREOF<br/>[FR] ACTIVATEURS DE LA VOIE DU GÈNE INDUCTIBLE PAR L'ACIDE RÉTINOÏQUE "RIG-I" ET LEURS PROCÉDÉS D'UTILISATION申请人:KINETA INC公开号:WO2020036574A1公开(公告)日:2020-02-20The present invention is directed to compounds of Formula (I), which are activators of the RIG-I pathway.本发明涉及式(I)化合物,该化合物是RIG-I通路的激活剂。
-
ACTIVATORS OF THE RETINOIC ACID INDUCIBLE GENE "RIG-I" PATHWAY AND METHODS OF USE THEREOF申请人:Kineta Immuno-Oncology LLC公开号:US20200055871A1公开(公告)日:2020-02-20The present invention is directed to compounds of Formula (I), which are activators of the RIG-I pathway.本发明涉及式(I)化合物,该化合物是RIG-I通路的激活剂。
-
AMINO ALCOHOL DERIVATIVE, PHARMACEUTICAL COMPOSITION AND APPLICATION THEREOF申请人:Beijing Foreland Pharma Co., Ltd.公开号:US20210347745A1公开(公告)日:2021-11-11The present invention belongs to the field of medicine, and specifically discloses an amino alcohol derivative represented by Formula I, a pharmaceutically acceptable salt, solvate, polymorph or prodrug thereof. In addition, the present invention also discloses a pharmaceutical composition comprising the above substances, and a use of the substance in the preparation of a medicament for the prevention and treatment of an immune inflammatory disease, or a disease or condition associated with immunological competence such as multiple sclerosis, ALS, CIDP, systemic lupus erythematosus, rheumatoid arthritis, ulcerative colitis, psoriasis, polymyositis, etc.
-
Design, synthesis, crystal structure, molecular docking studies of some diorganotin(IV) complexes derived from the piperonylic hydrazide Schiff base ligands as cytotoxic agents作者:Jai Devi、Jyoti Yadav、Kashmiri Lal、Nikhil Kumar、Avijit Kumar Paul、Deepak Kumar、Partha P. Dutta、Deepak Kumar JindalDOI:10.1016/j.molstruc.2021.129992日期:2021.5ligands having pentacoordinated geometry around the central tin metal. The X-ray crystallographic study of complex 9 (Me2SnL2) revealed the distorted trigonal bipyramidal geometry (SnO2NC2). The cytotoxic activity of compounds was tested against human cancer cell lines A549, Hela, MCF7 and normal cell line L6 using MTT assay. Compound 12 (Ph2SnL2), 14 (Et2SnL3), 19 (Bu2SnL4) was found most active against受有机锡(IV)配合物在抑制癌细胞生长以及与不同靶蛋白相互作用中的作用的启发,我们合成了一系列新的亚苄基苯并肼类似物席夫碱配体的有机锡(IV)配合物。通过光谱研究完成了化合物的结构阐明,光谱研究显示了在中心锡金属周围具有五配位几何形状的席夫碱配体的三齿性质(NOO)。配合物9(Me 2 SnL 2)的X射线晶体学研究揭示了扭曲的三角双锥体几何形状(SnO 2 NC 2)。使用MTT测定法测试了化合物对人癌细胞系A549,Hela,MCF7和正常细胞系L6的细胞毒活性。化合物发现12(Ph 2 SnL 2),14(Et 2 SnL 3),19(Bu 2 SnL 4)对测试的IC 50值为22.909-32.303μM的细胞系活性最高。此外,在酪氨酸激酶和EGFR激酶结构域的3D空间对活性化合物12、14和20进行了分子对接研究。
表征谱图
-
氢谱1HNMR
-
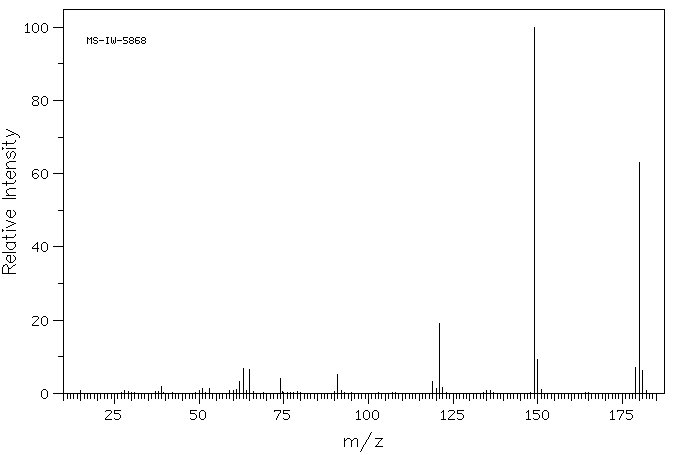
质谱MS
-
碳谱13CNMR
-
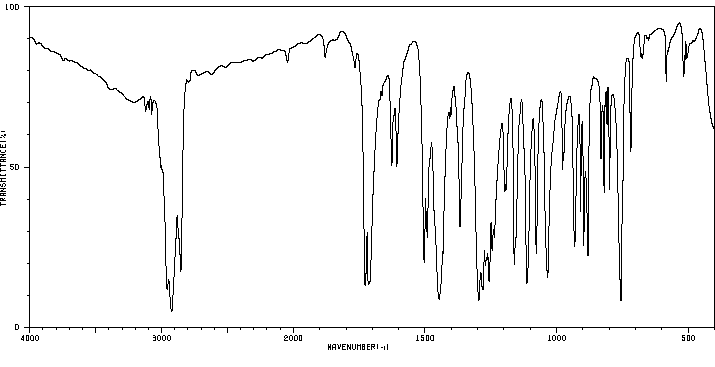
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5-(4-乙氧基-3-甲基苄基)-1,3-苯并二恶茂)
黄樟素氧化物
黄樟素乙二醇; 2',3'-二氢-2',3'-二羟基黄樟素
黄樟素
风藤酰胺
风藤酮
非哌西特盐酸盐
非哌西特 盐酸盐
角秋水仙碱
螺[1,3-苯并二氧戊环-2,1'-环己烷]-5-胺
蓝细菌
苯并[d][1,3]二氧杂环戊烯-5-胺盐酸盐
苯并[d][1,3]二氧代l-5-甲基(2-氧代乙基)氨基甲酸叔丁酯
苯并[d][1,3]二氧代l-5-氨基甲酸叔丁酯
苯并[d][1,3]二氧代-4-甲腈
苯并[d][1,3]二氧代-4-氨基甲酸叔丁酯
苯并[d[1,3]二氧代-4-羧酰胺
苯并[1,3]二氧杂环戊烯-5-基甲基2-氯乙酸酯
苯并[1,3]二氧杂环戊烯-5-基甲基-苄基-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-[2-(4-氟-苯基)-乙基]-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(四氢-呋喃-2-基甲基)-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(2-氟-苄基)-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(1-甲基-哌啶-4-基)-胺
苯并[1,3]二氧代l-5-甲基-吡啶-3-甲基-胺
苯并[1,3]二氧代l-5-甲基-(4-氟-苄基)-胺
苯并[1,3]二氧代l-5-乙酸甲酯
苯并[1,3]二氧代-5-羧酰胺盐酸盐
苯并[1,3]二氧代-5-甲基肼盐酸盐
苯并[1,3]二氧代-5-甲基吡啶-4-甲胺
苯并[1,3]二氧代-5-甲基-吡啶-2-甲胺
苯并[1,3]二氧代-5-乙酰氯
苯并-1,3-二氧杂环戊烯-5-甲醇丙酸酯
苯乙酸,1-(1,3-苯并二氧杂环戊烯-5-基)-3-丁烯-1-基酯
苯乙酮O-((4-(3,4-亚甲二氧基苄基)-1-哌嗪-1-基)羰基甲基)肟
苯,1-甲氧基-6-硝基-3,4-亚甲二氧基-
芝麻酚
芝麻林素
脲,N-1,3-苯并二噁唑-5-基-N'-(2-溴乙基)-
胡椒醛肟
胡椒醛-((Z)-O-苯基氨基甲酰基肟)
胡椒醛,二苄基缩硫醛
胡椒醛
胡椒醇
胡椒酸酰氯
胡椒酸
胡椒腈
胡椒环乙酮肟
胡椒环
胡椒基重氮酮
胡椒基甲醛