7-溴胡椒醛 | 19522-96-4
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:125 °C
-
沸点:152-153 °C
-
密度:1.782±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2932999099
-
WGK Germany:3
-
储存条件:应存放在室温、密封、干燥、避光、惰性气体环境中。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-溴-3, 4-二羟苯甲醛 3-bromo-4,5-dihydroxybenzaldehyde 16414-34-9 C7H5BrO3 217.019 胡椒醛 piperonal 120-57-0 C8H6O3 150.134 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 7-溴苯并[D][1,3]二氧戊环-5-羧酸 7-bromobenzo[d][1,3]-dioxole-5-carboxylic acid 66799-93-7 C8H5BrO4 245.029 —— methyl 7-bromobenzo[d][1,3]dioxole-5-carboxylate 103095-80-3 C9H7BrO4 259.056 —— N-methyl-5-bromopiperonylamine 212332-95-1 C9H10BrNO2 244.088 —— 7-bromo-5-(1,3-dioxolan-2-yl)-1,3-benzodioxole 312327-39-2 C10H9BrO4 273.083 —— 3-bromo-N-cyclohexyl-4,5-methylenedioxybenzylideneimine 81805-94-9 C14H16BrNO2 310.191 胡椒醛 piperonal 120-57-0 C8H6O3 150.134 —— (7-Bromo-benzo[1,3]dioxol-5-ylmethyl)-(2,2-diethoxy-ethyl)-methyl-amine 212332-86-0 C15H22BrNO4 360.248 7-羟基-1,3-苯并间二氧杂环戊烯-5-甲醛 5-hydroxypiperonal 81805-98-3 C8H6O4 166.133 —— 5-bromo-3,4-(methylenedioxy)phenol 66799-94-8 C7H5BrO3 217.019 —— 5-methoxy-2,3-methylenedioxybromobenzene 55950-26-0 C8H7BrO3 231.046 肉豆蔻醛 3-Methoxy-4,5-methylenedioxybenzaldehyde 5780-07-4 C9H8O4 180.16 —— 3-[2H3]methoxy-4,5-(methylenedioxy)benzaldehyde 1037620-82-8 C9H8O4 183.136 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Kondo et al., Itsuu Kenkyusho Nempo, 1950, # 1, p. 15,16; engl. Ref. S. 54, 55摘要:DOI:
-
作为产物:描述:参考文献:名称:Selective and Efficient Oxidation of Benzylic Alcohols to Benzaldehydes and Methyl Benzoates by Dibromo-5,5-dimethylhydantoin摘要:A selective and efficient method of oxidizing benzyl alcohols to benzaldehydes and methyl benzoates by using 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) as oxidant is developed. One-step conversion of benzyl alcohols to methyl benzoates in methanol at room temperature for 12 hours is achieved without any catalysts. Moreover, para-substituted benzyl alcohols are obtained in 86-98% yield. When dichloromethane is used as solvent, further oxidation of benzaldehydes to esters is well controlled, selectively affording benzaldehydes in 89-99% yield within 30 minutes.[Supplementary materials are available for this article. Go to the publisher's online edition of Synthetic Communications (R) for the following free supplemental resource(s): Full experimental and spectral details.]DOI:10.1080/00397911.2013.856447
文献信息
-
Heterocyclic derivatives for the treatment of cancer and other proliferative diseases申请人:——公开号:US20020143182A1公开(公告)日:2002-10-03The invention relates to certain heterocyclic compounds useful for the treatment of cancer and other diseases, having the Formula (I): 1 wherein: (a) m is an integer 0 or 1; (b) R 12 is an alkyl, a substituted alkyl, a cycloalkyl, a substituted cycloalkyl, a heterocyclic, a substituted heterocyclic, a heteroaryl, a substituted heteroaryl, an aryl or a substituted aryl residue; (c) Ar 3 is an aryl, a substituted aryl, a heteroaryl or a substituted heteroaryl residue; (d) Ar 4 is an aryl, a substituted aryl, a heteroaryl or a substituted heteroaryl residue; (e) R 5 is hydrogen, hydroxy, alkyl or substituted alkyl; (f) - - - - - represents a bond present or absent; and (g) W, X, Y and Z are independently or together C(O)—, C(S), S, O, or NH; or a pharmaceutically acceptable salt thereof.该发明涉及某些对治疗癌症和其他疾病有用的杂环化合物,其具有以下式(I): 1 其中: (a) m是整数0或1; (b) R12是烷基,取代烷基,环烷基,取代环烷基,杂环基,取代杂环基,杂芳基,取代杂芳基,芳基或取代芳基残基; (c) Ar3是芳基,取代芳基,杂芳基或取代杂芳基残基; (d) Ar4是芳基,取代芳基,杂芳基或取代杂芳基残基; (e) R5是氢,羟基,烷基或取代烷基; (f) - - - - - 代表存在或不存在的键;以及 (g) W、X、Y和Z独立或一起是C(O)、C(S)、S、O或NH;或其药学上可接受的盐。
-
Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents作者:B.Shivarama Holla、K.V. Malini、B.Sooryanarayana Rao、B.K. Sarojini、N.Suchetha KumariDOI:10.1016/s0223-5234(02)01447-2日期:2003.3A series of arylthioureas (1), aromatic aldehyde thiosemicarbazones (2) and 5-aryl-2-furfuraldehyde thiosemicarbazones (3) were condensed with 2,4-dichloro-5-fluorophenacyl bromide to yield respective arylaminothiazoles, arylidene/5-aryl-2-furfurylidene hydrazinothiazoles (4). The newly synthesized compounds were characterized by IR, 1H-NMR and mass spectral studies. These compounds were also screened
-
[EN] MACROCYCLIC COMPOUNDS USEFUL AS PHARMACEUTICALS<br/>[FR] COMPOSES MACROCYCLIQUES UTILES COMME PRODUITS PHARMACEUTIQUES申请人:EISAI CO LTD公开号:WO2003076424A1公开(公告)日:2003-09-18The present invention provides compounds having formula (I), and additionally provides methods for the synthesis thereof and methods for the use thereof in the treatment of various disorders including inflammatory or autoimmune disorders, and disorders involving malignancy or increased angiogenesis, wherein R1 -R11, t, X, Y, Z, and n are as defined herein.本发明提供具有式(I)的化合物,并另外提供其合成方法以及在治疗包括炎症性或自身免疫性疾病、恶性肿瘤或增加血管生成等各种疾病中使用的方法,其中R1-R11、t、X、Y、Z和n如本文所定义。
-
Direct Substitution of Arylalkynyl Carbinols Provides Access to Diverse Terminal Acetylene Building Blocks作者:Narendran G-Dayanandan、Eric W. Scocchera、Santosh Keshipeddy、Heather F. Jones、Amy C. Anderson、Dennis L. WrightDOI:10.1021/acs.orglett.6b03438日期:2017.1.6trimethoprim-resistant bacteria, synthetic methods were needed to prepare a diverse array of 3-aryl-propynes with various substitutions at the propargyl position. A direct route was sought whereby nucleophilic addition of acetylene to aryl carboxaldehydes would be followed by reduction or substitution of the resulting propargyl alcohol. The direct reduction, methylation, and dimethylation of these readily available
-
Design and Microwave Synthesis of New (5Z) 5-Arylidene-2-thioxo-1,3-thiazolinidin-4-one and (5Z) 2-Amino-5-arylidene-1,3-thiazol-4(5H)-one as New Inhibitors of Protein Kinase DYRK1A作者:Khadidja Bourahla、Solène Guihéneuf、Emmanuelle Limanton、Ludovic Paquin、Rémy Le Guével、Thierry Charlier、Mustapha Rahmouni、Emilie Durieu、Olivier Lozach、François Carreaux、Laurent Meijer、Jean-Pierre BazureauDOI:10.3390/ph14111086日期:——
Here, we report on the synthesis of libraries of new 5-arylidene-2-thioxo-1,3-thiazolidin-4-ones 3 (twenty-two compounds) and new 2-amino-5-arylidene-1,3-thiazol-4(5H)-ones 5 (twenty-four compounds) with stereo controlled Z-geometry under microwave irradiation. The 46 designed final compounds were tested in order to determine their activity against four representative protein kinases (DYR1A, CK1, CDK5/p25, and GSK3α/β). Among these 1,3-thiazolidin-4-ones, the molecules (5Z) 5-(4-hydroxybenzylidene)-2-thioxo-1,3-thiazolidin-4-one 3e (IC50 0.028 μM) and (5Z)-5-benzo[1,3]dioxol-5-ylmethylene-2-(pyridin-2-yl)amino-1,3-thiazol-4(5H)-one 5s (IC50 0.033 μM) were identified as lead compounds and as new nanomolar DYRK1A inhibitors. Some of these compounds in the two libraries have been also evaluated for their in vitro inhibition of cell proliferation (Huh7 D12, Caco2, MDA-MB 231, HCT 116, PC3, and NCI-H2 tumor cell lines). These results will enable us to use the 1,3-thiazolidin-4-one core as pharmacophores to develop potent treatment for neurological or oncological disorders in which DYRK1A is fully involved.
在这里,我们报道了在微波辐射下合成了新的5-芳基亚基-2-硫代-1,3-噻唑啉-4-酮3类(二十二个化合物)和新的2-氨基-5-芳基亚基-1,3-噻唑-4(5H)-酮5类(二十四个化合物),具有立体控制的Z-几何构型。设计的46个最终化合物被测试,以确定它们对四种代表性蛋白激酶(DYR1A、CK1、CDK5/p25和GSK3α/β)的活性。在这些1,3-噻唑啉-4-酮中,分子(5Z)5-(4-羟基苯基亚基)-2-硫代-1,3-噻唑啉-4-酮3e(IC50为0.028μM)和(5Z)-5-苯并[1,3]二氧杂环-5-基亚甲基-2-(吡啶-2-基)氨基-1,3-噻唑-4(5H)-酮5s(IC50为0.033μM)被确定为引物化合物和新的纳摩尔级DYRK1A抑制剂。这两个库中的一些化合物也已评估其对细胞增殖的体外抑制作用(Huh7 D12、Caco2、MDA-MB 231、HCT 116、PC3和NCI-H2肿瘤细胞系)。这些结果将使我们能够利用1,3-噻唑啉-4-酮核作为药效团,开发用于治疗DYRK1A完全参与的神经或肿瘤疾病的有效治疗方法。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
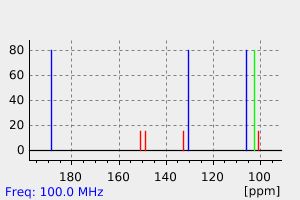
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息