烯丙基丙二酸二乙酯 | 2049-80-1
中文名称
烯丙基丙二酸二乙酯
中文别名
二乙基丙烯基丙二酸酯;二乙基烯丙基丙二酸酯
英文名称
(2-propenyl)propanedioic acid diethyl ester
英文别名
Diethyl allylmalonate;diethyl 2-allylmalonate;diethyl 2-prop-2-enylpropanedioate
CAS
2049-80-1
化学式
C10H16O4
mdl
MFCD00009155
分子量
200.235
InChiKey
GDWAYKGILJJNBB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:222-223 °C(lit.)
-
密度:1.015 g/mL at 25 °C(lit.)
-
闪点:160 °F
-
LogP:1.990 (est)
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,也没有已知的危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:14
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29171990
-
危险品运输编号:200kgs
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境具有良好的通风或排气设施。
SDS
1.1 产品标识符
: 二乙基烯丙基丙二酸酯
产品名称
1.2 鉴别的其他方法
Allylmalonic acid diethyl ester
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
易燃液体 (类别4)
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H227 可燃液体
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P210 远离热源、火花、明火和热表面。- 禁止吸烟。
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P403 + P235 存放在通风良好的地方。保持低温。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Allylmalonic acid diethyl ester
别名
: C10H16O4
分子式
: 200.23 g/mol
分子量
组分 浓度或浓度范围
Diethyl allylmalonate
-
CAS 号 2049-80-1
EC-编号 218-072-9
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
禁止催吐。 切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
小(起始)火时,使用媒介物如“乙醇”泡沫、干化学品或二氧化碳。大火时,尽可能使用水灭火。使用大量(
洪水般的)水以喷雾状应用;水柱可能是无效的。用大量水降温所有受影响的容器。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
水喷雾可用来冷却未打开的容器。
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 移去所有火源。
将人员撤离到安全区域。 防范蒸汽积累达到可爆炸的浓度,蒸汽能在低洼处积聚。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用防电真空清洁器或湿的刷子将溢出物收集起来并放置到容器中去,根据当地规定处理(见第13部分)。
存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
切勿靠近火源。-严禁烟火。采取措施防止静电积聚。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 淡黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
222 - 223 °C - lit.
g) 闪点
71 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
1.015 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
热,火焰和火花。
10.5 不兼容的材料
酸, 碱, 氧化剂, 还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
此易爆炸产品可以在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 二乙基烯丙基丙二酸酯
产品名称
1.2 鉴别的其他方法
Allylmalonic acid diethyl ester
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
易燃液体 (类别4)
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H227 可燃液体
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P210 远离热源、火花、明火和热表面。- 禁止吸烟。
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P403 + P235 存放在通风良好的地方。保持低温。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Allylmalonic acid diethyl ester
别名
: C10H16O4
分子式
: 200.23 g/mol
分子量
组分 浓度或浓度范围
Diethyl allylmalonate
-
CAS 号 2049-80-1
EC-编号 218-072-9
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
禁止催吐。 切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
小(起始)火时,使用媒介物如“乙醇”泡沫、干化学品或二氧化碳。大火时,尽可能使用水灭火。使用大量(
洪水般的)水以喷雾状应用;水柱可能是无效的。用大量水降温所有受影响的容器。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
水喷雾可用来冷却未打开的容器。
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 移去所有火源。
将人员撤离到安全区域。 防范蒸汽积累达到可爆炸的浓度,蒸汽能在低洼处积聚。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用防电真空清洁器或湿的刷子将溢出物收集起来并放置到容器中去,根据当地规定处理(见第13部分)。
存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
切勿靠近火源。-严禁烟火。采取措施防止静电积聚。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 淡黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
222 - 223 °C - lit.
g) 闪点
71 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
1.015 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
热,火焰和火花。
10.5 不兼容的材料
酸, 碱, 氧化剂, 还原剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
此易爆炸产品可以在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— triethyl pent-4-ene-1,2,2-tricarboxylate 16515-85-8 C13H20O6 272.298 二烯丙基丙二酸二乙酯 Diethyl diallylmalonate 3195-24-2 C13H20O4 240.299 —— diethyl 2-allyl-2-bromomalonate 78331-59-6 C10H15BrO4 279.131 烯丙基丙二酸 allylmalonic acid 2583-25-7 C6H8O4 144.127 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(ethoxycarbonyl)pent-4-enoic acid 2985-38-8 C8H12O4 172.181 —— (E)-diethyl (but-2-en-1-yl)malonate 84200-37-3 C11H18O4 214.262 —— diisopropyl 2-allylmalonate —— C12H20O4 228.288 (3-甲基丁-2-烯基)丙二酸二乙酯 diethyl (3-methylbut-2-enyl)malonate 22539-80-6 C12H20O4 228.288 —— diethyl 2-[3-cyano-2-propenyl]malonate —— C11H15NO4 225.244 —— diethyl 2-[(E)-3-cyano-2-propenyl]malonate —— C11H15NO4 225.244 —— diethyl 2-[(E)-3-cyano-2-propenyl]malonate —— C11H15NO4 225.244 2-烯丙基-2-甲基丙二酸二乙酯 diethyl allylmethylmalonate 53651-72-2 C11H18O4 214.262 —— 2-(2-methyl-allyl)-malonic acid diethyl ester 1575-67-3 C11H18O4 214.262 —— (E)-1,1-diethyl 4-methyl but-3-ene-1,1,4-tricarboxylate 579503-43-8 C12H18O6 258.271 —— diethyl 2-(2'-chloroprop-2'-en-1'-yl)-1,3-propandioate 22426-62-6 C10H15ClO4 234.68 —— diethyl (E)-2-(4,6-dimethylhepta-2,5-dienyl)malonate 1418144-06-5 C16H26O4 282.38 2-烯丙基-2-甲氧基甲基丙二酸二乙酯 diethyl 2-allyl-2-methoxymethylmalonate 959839-04-4 C12H20O5 244.288 2-烯丙基-2-乙基丙二酸二乙酯 diethyl allylethylmalonate 59726-37-3 C12H20O4 228.288 —— triethyl pent-4-ene-1,2,2-tricarboxylate 16515-85-8 C13H20O6 272.298 二烯丙基丙二酸二乙酯 Diethyl diallylmalonate 3195-24-2 C13H20O4 240.299 —— diethyl 2-[(2E,5E)-6,10-dimethylundeca-2,5,9-trienyl]malonate 1418143-86-8 C20H32O4 336.472 丙基丙二酸二乙酯 diethyl propylmalonate 2163-48-6 C10H18O4 202.251 —— 2-(ethoxycarbonyl)-2-ethylpent-4-enoic acid 94489-12-0 C10H16O4 200.235 3-环戊烯-1,1-二甲酸二乙酯 Cyclopent-3-ene-1,1-dicarboxylic acid diethyl ester 21622-00-4 C11H16O4 212.246 —— 3-Ethoxy-2-allyl-propionsaeure 90113-65-8 C8H14O3 158.197 —— diethyl 2-(hex-5-en-1-yl)malonate 69298-59-5 C13H22O4 242.315 —— Diethyl 2-(4-oxobutyl)propanedioate 175663-10-2 C11H18O5 230.261 —— diethyl 2-allyl-2-(but-2-en-1-yl)malonate —— C14H22O4 254.326 —— diethyl (but-2'-en-1'-yl)(prop-2''-en-1''-yl)propanedioate 112586-60-4 C14H22O4 254.326 3-氯丙基丙二酸二乙酯 diethyl (3-chloropropyl)malonate 18719-43-2 C10H17ClO4 236.696 —— diethyl 2-[(2E,5Z)-5-ethylidene-9-methyldeca-2,8-dienyl]malonate 1418143-87-9 C20H32O4 336.472 (3-溴丙基)丙二酸二乙酯 diethyl 2-(3-bromopropane)malonate 10149-21-0 C10H17BrO4 281.147 —— 2-methyl-4,4-diethoxycarbonylbutanal 87879-73-0 C11H18O5 230.261 —— diethyl (4-bromobutyl)malonate 132525-49-6 C11H19BrO4 295.173 —— diethyl 2-allyl-2-(penta-2,3-dien-1-yl)malonate 1304145-47-8 C15H22O4 266.337 —— diethyl 2-allyl-2-[(2Z)-4-hydroxybut-2-enyl]malonate 195323-61-6 C14H22O5 270.326 —— diethyl (E)-cinnamyl malonate 63082-55-3 C16H20O4 276.332 —— diethyl 2-allyl-2-isopropylmalonate 59726-39-5 C13H22O4 242.315 —— 2-(4,4-Difluoro-but-3-enyl)-malonic acid diethyl ester 168901-96-0 C11H16F2O4 250.242 2-丙烯基丙基丙二酸二乙酯 diethyl 2-propyl-2-allyl-1,3-propanedioate 59726-38-4 C13H22O4 242.315 —— diethyl 2-(3-methyl-3-buten-1-yl)malonate 176376-86-6 C12H20O4 228.288 —— diethyl 2-allyl-2-(4-oxobut-2-enyl)malonate 349082-67-3 C14H20O5 268.31 —— 2-(3-methyl-2-buten-1-yl)-2-(2-propen-1-yl)malonic acid, diethyl ester 408333-38-0 C15H24O4 268.353 —— 2-(3-Methylsulfanyl-propyl)-malonic acid diethyl ester 28438-54-2 C11H20O4S 248.343 —— Diethyl 2-(2-chloroethyl)-2-prop-2-enylpropanedioate 174619-41-1 C12H19ClO4 262.733 —— diethyl 2-allyl-2-[(E)-4-bromo-2-butenyl]-1,3-propanedioate 879098-77-8 C14H21BrO4 333.222 —— diethyl 2-(5-methyl-2-methylene-4-hexenyl)malonate —— C15H24O4 268.353 —— diethyl 2-allyl-2-(2-iodoethyl)malonate —— C12H19IO4 354.185 2-烯丙基-2-(2-丙炔基)丙二酸二乙酯 4,4-bis(ethoxycarbonyl)-1-hepten-6-yne 101268-55-7 C13H18O4 238.284 —— Diethyl 2-(3,3-dideuterioprop-2-enyl)-2-prop-2-enylpropanedioate 1228236-01-8 C13H20O4 242.284 —— diethyl 2-allyl-2-(2-bromoethyl)malonate 174619-42-2 C12H19BrO4 307.184 —— diethyl 2-chloro-2-(2-propenyl)-propanedioate 100131-32-6 C10H15ClO4 234.68 —— diethyl allylfluoromalonate 1775-48-0 C10H15FO4 218.225 —— diethyl 2-(2-methoxyethyl)-2-allylmalonate 188125-24-8 C13H22O5 258.315 —— 5-allyl-2,2-dimethyl-1,3-dioxan-4,6-dione 113427-02-4 C9H12O4 184.192 —— 2-allyl-2-but-3-enyl-malonic acid diethyl ester 51481-46-0 C14H22O4 254.326 —— (E)-diethyl 2-allyl-2-(4-methylpenta-2,4-dien-1-yl)malonate 1647100-78-4 C16H24O4 280.364 —— dimethyl 2-allyl-2-(2-cyclopropylidenethyl)malonate 875737-22-7 C15H22O4 266.337 —— diethyl 2-allyl-2-bromomalonate 78331-59-6 C10H15BrO4 279.131 烯丙基异丁基丙二酸二乙酯 diethyl 2-isobutyl-2-(prop-2-enyl)malonate 59726-40-8 C14H24O4 256.342 —— 2-butyl-2-ethoxycarbonylpenten-4-oic acid ethyl ester 1575-76-4 C14H24O4 256.342 —— diethyl 2-[(4Z)-4-methyl-2-methylene-4-hexenyl]malonate —— C15H24O4 268.353 —— Methyl 5,5-Bis(ethoxycarbonyl)-2,7-octadienoate 143925-27-3 C15H22O6 298.336 —— diethyl 2-allyl-2-(pent-4-en-1-yl)malonate —— C15H24O4 268.353 —— allyl-nonyl-malonic acid diethyl ester 176376-79-7 C19H34O4 326.477 2-氯丙基丙二酸二乙酯 diethyl 2-chloropropylmalonate 104643-72-3 C10H17ClO4 236.696 (3-甲基丁基)烯丙基丙二酸二乙酯 diethyl 2-(3-methylbutyl)-2-(propen-2-yl)malonate 59726-44-2 C15H26O4 270.369 —— diethyl allyl(methallyl)malonate 5309-50-2 C14H22O4 254.326 二乙基甲基(4-戊烯基)丙二酸酯 Diethyl methyl(4-pentenyl)malonate 50317-20-9 C13H22O4 242.315 —— 2-(3-Trichlorosilanyl-propyl)-malonic acid diethyl ester 22408-99-7 C10H17Cl3O4Si 335.687 —— diethyl 7-octen-2-yne-5,5-dicarboxylate 119548-53-7 C14H20O4 252.31 2-甲基-4-戊烯酸乙酯 ethyl 2-methyl-4-pentenoate 53399-81-8 C8H14O2 142.198 —— diethyl 2-(4,5-dimethyl-2-methylene-4-hexenyl)malonate —— C16H26O4 282.38 —— diethyl (2-chloroprop-2-en-1-yl)(prop-2-en-1-yl)propandioate 575501-24-5 C13H19ClO4 274.744 —— diethyl 2-(4'-bromobutyl)(prop-2''-en-1''-yl)propanedioate 764583-44-0 C14H23BrO4 335.238 —— diethyl 2-(2-bromo-2-propenyl)-2-(2-propenyl)propanedioate 118453-14-8 C13H19BrO4 319.195 —— diethyl 2-allyl-2-(hept-2-yn-1-yl)malonate 191801-57-7 C17H26O4 294.391 —— diethyl 8-nonen-3-yne-6,6-dicarboxylate 101101-24-0 C15H22O4 266.337 —— diethyl allyl(2-carboxyethyl)malonate 176376-83-3 C13H20O6 272.298 —— allyl-sec-butyl-malonic acid diethyl ester 59726-41-9 C14H24O4 256.342 —— cyclohex-3-ene-1,1-dicarboxylic acid diethyl ester 38511-09-0 C12H18O4 226.273 —— (2-octynyl)-(2-propenyl)propanedioic acid diethyl ester 350248-68-9 C18H28O4 308.418 —— 2-(4-Chloro-4,4-difluoro-butyl)-malonic acid diethyl ester 168901-97-1 C11H17ClF2O4 286.703 四乙基十二碳-1-烯-6,11-二炔-3,3,8,8-四羧酸酯 4,4,9,9-tetra(carbethoxy)dodec-11-ene-1,6-diyne 264873-23-6 C24H32O8 448.513 —— diethyl 2-allyl-2-(4-hydroxybut-2-ynyl)malonate 904285-40-1 C14H20O5 268.31 —— 3,3-bis(ethoxycarbonyl)hex-5-enoic acid 193806-55-2 C12H18O6 258.271 —— allyl-cyclobutylmethyl-malonic acid diethyl ester 411238-98-7 C15H24O4 268.353 —— diethyl 2-allyl-2-(3-methylbut-3-en-1-yl)malonate 176376-85-5 C15H24O4 268.353 —— 5,5-dicarboethoxy-7-octen-2-on 92746-99-1 C14H22O5 270.326 —— diisopropyl 2-allyl-2-(but-2-yn-1-yl)malonate —— C16H24O4 280.364 —— diethyl (3-triethylsilylpropyl)malonate 22408-93-1 C16H32O4Si 316.513 —— diethyl 2-oxo-6-heptene-4,4-dicarboxylate 164596-08-1 C13H20O5 256.299 —— ethyl 4-(diethylmaleonate)propylphosphinate 1443525-46-9 C12H23O6P 294.285 —— Methoxymethyl-allyl-malonsaeure 90482-58-9 C8H12O5 188.18 —— ethyl (±)-4,5-epoxy-2-(ethoxycarbonyl)pentanoate 13353-23-6 C10H16O5 216.234 —— 3-O,3-O-diethyl 1-O-prop-2-enyl hex-5-ene-1,3,3-tricarboxylate 176376-82-2 C16H24O6 312.363 —— 3-methyl-cyclopent-3-ene-1,1-dicarboxylic acid diethyl ester 2698-64-8 C12H18O4 226.273 —— 9,9-bis(carbethoxy)-4-oxadodec-11-ene-1,6-diyne 873800-55-6 C17H22O5 306.359 —— 4,4-bis(carbethoxy)-9-oxatridec-1-ene-6,11-diyne 873800-72-7 C18H24O5 320.386 (1-甲基丁基)烯丙基丙二酸二乙酯 allyl-(1-methyl-butyl)-malonic acid diethyl ester 6285-59-2 C15H26O4 270.369 2-乙基-4-戊烯酸乙酯 ethyl 2-ethyl-4-pentenoate 67030-95-9 C9H16O2 156.225 烯丙基丙二酸 allylmalonic acid 2583-25-7 C6H8O4 144.127 —— 2-(2-bromo-ethyl)-2-(4-oxo-pent-2-enyl)-malonic acid diethyl ester —— C14H21BrO5 349.222 —— 4,4-dicarboethoxy-6-heptenic acid chloride 176376-84-4 C13H19ClO5 290.744 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:芳氧基钛 (IV) 催化 1,6-和 1,7-二烯的环化反应摘要:DOI:10.1021/ja9922680
-
作为产物:描述:triethyl pent-4-ene-1,2,2-tricarboxylate 在 sodium ethanolate 作用下, 以 四氢呋喃 为溶剂, 反应 1.0h, 以87%的产率得到烯丙基丙二酸二乙酯参考文献:名称:铁催化、氢介导的 1,6-烯炔和二炔还原环化:双(亚氨基)吡啶配体参与的证据摘要:双(亚氨基)吡啶铁二氮配合物(((i)Pr)PDI)Fe(N(2))(2)催化氢介导的烯炔和二炔的还原环化,其周转频率与已建立的贵金属催化剂相当. 氨基、含氧和碳基底物很容易环化为相应的杂环和碳环,在 23 摄氏度时含有 5 mol % 铁和 4 atm H(2)。选定的底物与 N(2) 下的铁化合物之间的化学计量反应) 气氛建立了从异丙基芳基取代基到烯炔或二炔底物的转移脱氢。通过 (1) H NMR 光谱结合氘标记实验对催化反应进行原位监测,建立了快速环化,然后是限制营业额的氢化。DOI:10.1021/ja902478p
-
作为试剂:描述:5-bromo-5-nitro-2-phenyl-1,3-dioxane 在 乙醚 、 烯丙基丙二酸二乙酯 、 sodium-compound 作用下, 生成 2-phenyl-5-nitro-1,3-dioxane参考文献:名称:Eckstein, Roczniki Chemii, 1956, vol. 30, p. 1151,1153, 1156摘要:DOI:
文献信息
-
Bifunctional Iminophosphorane Catalyzed Enantioselective Sulfa-Michael Addition to Unactivated α-Substituted Acrylate Esters作者:Alistair J. M. Farley、Christopher Sandford、Darren J. DixonDOI:10.1021/jacs.5b10226日期:2015.12.30The highly enantioselective sulfa-Michael addition of alkyl thiols to unactivated α-substituted acrylate esters catalyzed by a bifunctional iminophosphorane organocatalyst under mild conditions is described. The strong Brønsted basicity of the iminophosphorane moiety of the catalyst provides the necessary activation of the alkyl thiol pro-nucleophile, while the two tert-leucine residues flanking a
-
Divergent C–H Oxidative Radical Functionalization of Olefins to Install Tertiary Alkyl Motifs Enabled by Copper Catalysis作者:Ming-Qing Tian、Cong Wang、Xu-Hong Hu、Teck-Peng LohDOI:10.1021/acs.orglett.9b00142日期:2019.3.15An efficient tertiary alkylation reaction of olefins with 1,3-dicarbonyl compounds was developed by virtue of copper catalyst without the use of expensive ligands or additives. In contrast to alkyl Heck-type reaction, alkyl halide is not required. Notably, by varying the nitrogen and air atmosphere, the reaction selectively produces alkylation and alkylation–oxygenation products, respectively. Initial
-
A NEW SYNTHESIS OF <scp>DL</scp>-γ-HYDROXY-ORNITHINE作者:Guy Talbot、Roger Gaudry、Louis BerlinguetDOI:10.1139/v56-120日期:1956.7.1
A convenient synthesis of DL-hydroxy-ornithine is described. Starting from diethyl allylmalonate, it involves treatment with sulphuryl chloride followed by hydrolysis and distillation to give a 90% yield of 2,5-dichloro-4-valerolactone. Condensation of this with two equivalents of potassium phthalimide in dimethyl-formamide gives a quantitative yield of crude 2,5-diphthalimido-4-valerolactone. This lactone is converted quantitatively by acid hydrolysis to DL-γ-hydroxyornithine, isolated as the dihydrochloride of the corresponding 2,5-diamino-4-valerolactone. The over-all yield calculated from allyl chloride is 80%.
-
SeO<sub>2</sub>-Mediated Oxidative Transposition of Pauson–Khand Products作者:Sara E. Dibrell、Michael R. Maser、Sarah E. ReismanDOI:10.1021/jacs.9b13818日期:2020.4.8Oxidative transpositions of bicyclic cyclopentenones mediated by selenium dioxide (SeO2) are disclosed. Treatment of Pauson-Khand reaction (PKR) products with SeO2 in the presence or absence of water furnishes di- and trioxidized cyclopentenones, respectively. Mechanistic investigations reveal multiple competing oxidation pathways that depend on substrate identity and water concentration. Functionalization
-
Allylation of Active Methylene Compounds with Allyl Oxime Carbonates Catalyzed by Pd(0)作者:Osamu Suzuki、Yoshiharu Hashiguchi、Seiichi Inoue、Kikumasa SatoDOI:10.1246/cl.1988.291日期:1988.2.5Allylation of active methylene compounds catalyzed by a palladium(0)-phosphine system took place highly stereoselectively by employing allyl oxime carbonates as the allylating reagent.
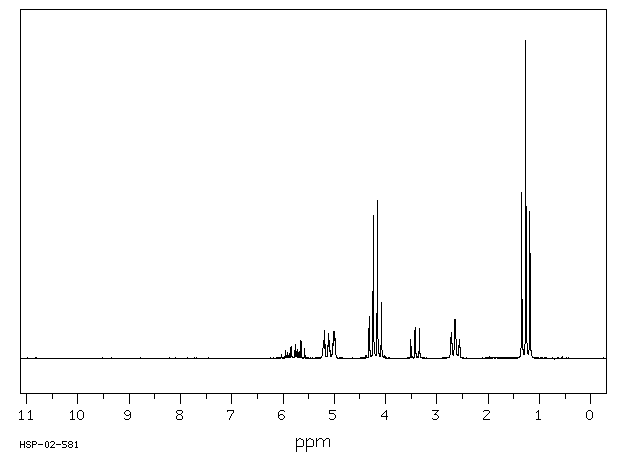
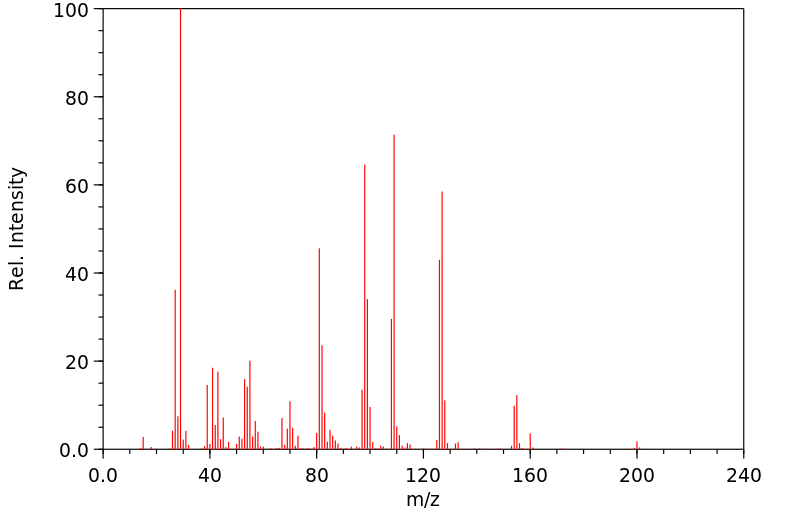
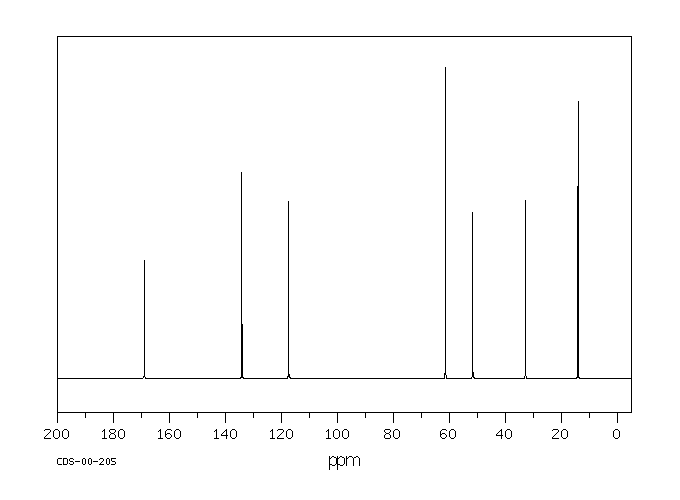
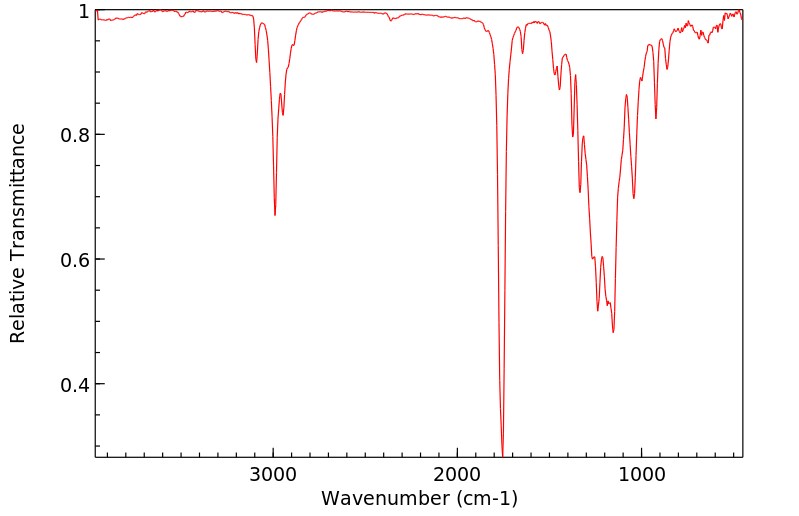
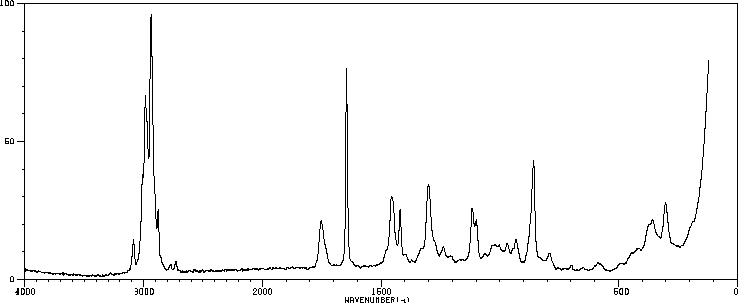
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯