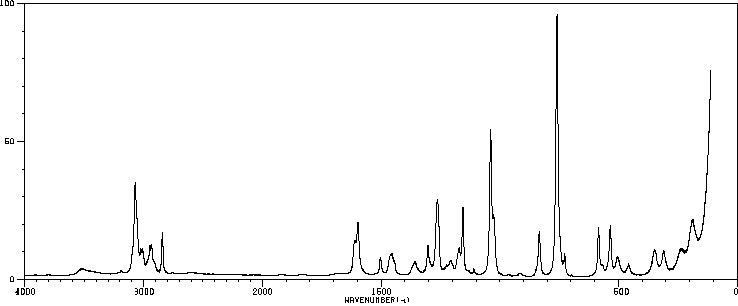
代谢
Several strains of Aspergillus niger hydroxylated anisole to give o-hydroxyanisole as main product.
来源:Hazardous Substances Data Bank (HSDB)