2-硝基苯甲醚 | 91-23-6
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:9-12 °C (lit.)
-
沸点:273 °C (lit.)
-
密度:1.254 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
溶解度:酒精:可溶(lit.)
-
介电常数:43.0
-
物理描述:COLOURLESS-TO-YELLOW-RED LIQUID.
-
颜色/状态:Colorless to yellowish liquid
-
蒸汽密度:Relative vapor density (air = 1): 5.29
-
蒸汽压力:3.6X10-3 mm Hg at 25 °C
-
自燃温度:464 °C
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxide/.
-
粘度:Liquid viscosity = 3.9768X10-3 at melting point
-
表面张力:4.7471X10-2 N/m at melting point
-
折光率:Index of refraction = 1.5161 at 20 °C/D
-
保留指数:1296
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:55
-
氢给体数:0
-
氢受体数:3
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
安全说明:S45,S53
-
危险品运输编号:UN 2730 6.1/PG 3
-
WGK Germany:3
-
海关编码:29093090
-
危险类别:6.1
-
危险品标志:T
-
危险类别码:R22,R45
-
RTECS号:BZ8790000
-
包装等级:III
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,保持容器密封。 - 应与氧化剂、还原剂、酸类、碱类、食用化学品分开存放,切忌混储。 - 配备相应品种和数量的消防器材。 - 储区应备有泄漏应急处理设备和合适的收容材料。
制备方法与用途
化学性质
这是一种无色至浅黄色的易燃液体,能溶于乙醇和乙醚,但不溶于水。
用途
该物质广泛应用于染料、医药及香料等工业领域,可用于生产邻氨基苯甲醚、联大茴香胺、色酚AS-OL、大红色基B、直接湖蓝6B、活性溶蓝KD-7G、净洗剂LS等多种产品。
生产方法
其制备过程包括将邻硝基氯苯与烧碱和甲醇进行甲氧基化反应,生成粗品后经过蒸馏、洗涤及干燥处理,最终得到成品。原料消耗定额为:每吨需用邻硝基氯苯1120千克、甲醇270千克。
类别
有毒物品
毒性分级
中毒
急性毒性
口服 - 大鼠 LD50: 740 毫克/公斤;小鼠 LD50: 1300 毫克/公斤
可燃性危险特性
遇明火易燃,并会产生有毒氮氧化物烟雾。
储运特性
应存放在通风、低温且干燥的库房中,避免与氧化剂或食品添加剂混放。
职业标准
短期暴露极限(STEL)为1毫克/立方米。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 邻硝基苯乙醚 1-ethoxy-2-nitrobenzene 610-67-3 C8H9NO3 167.164 4-甲氧基-3-硝基苯胺 4-methoxy-3-nitroaniline 577-72-0 C7H8N2O3 168.152 硝苯酚 2-hydroxynitrobenzene 88-75-5 C6H5NO3 139.111 2,4-二硝基苯甲醚 2,4-dinitroanisole 119-27-7 C7H6N2O5 198.135 4-碘-2-硝基苯甲醚 4-iodo-2-nitroanisole 52692-09-8 C7H6INO3 279.034 1-甲氧基-2-亚硝基苯 2-nitrosoanisole 17075-26-2 C7H7NO2 137.138 4-甲氧基-3-硝基苯硼酸 4-methoxy-3-nitrophenylboronic acid 827614-67-5 C7H8BNO5 196.955 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 邻硝基苯乙醚 1-ethoxy-2-nitrobenzene 610-67-3 C8H9NO3 167.164 3-甲氧基-4-硝基苯胺 3-methoxy-4-nitroaniline 16292-88-9 C7H8N2O3 168.152 3-甲氧基-4-硝基苯酚 4-nitro-3-methoxyphenol 16292-95-8 C7H7NO4 169.137 —— 2-allyloxy-1-nitrobenzene 55339-51-0 C9H9NO3 179.175 1-硝基-2-丙氧基苯 1-nitro-2-propoxybenzene 3079-53-6 C9H11NO3 181.191 1-硝基-2-丙-2-基氧基苯 1-isopropoxy-2-nitrobenzene 38753-50-3 C9H11NO3 181.191 4-甲氧基-3-硝基苯胺 4-methoxy-3-nitroaniline 577-72-0 C7H8N2O3 168.152 1-(2-甲基丙氧基)-2-硝基苯 1-isobutoxy-2-nitrobenzene 56245-02-4 C10H13NO3 195.218 1-丁氧基-2-硝基苯 n-butyl o-nitrophenyl ether 7252-51-9 C10H13NO3 195.218 1-叔丁氧基-2-硝基苯 1-tert-butoxy-2-nitrobenzene 83747-12-0 C10H13NO3 195.218 2-硝基苯基戊基醚 1-nitro-2-(pentyloxy)benzene 39645-91-5 C11H15NO3 209.245 —— 1-(2-(2-methoxyethoxy)ethoxy)-2-nitrobenzene 136814-65-8 C11H15NO5 241.244 2,6-二硝基苯甲醚 2,6-dinitroanisole 3535-67-9 C7H6N2O5 198.135 O-(2-甲基烯丙基氧基)硝基苯 2-[(2-methyl-2-propenyl)oxy]-1-nitrobenzene 13414-54-5 C10H11NO3 193.202 硝苯酚 2-hydroxynitrobenzene 88-75-5 C6H5NO3 139.111 4-氯-2-硝基苯甲醚 4-chloro-2-nitroanisole 89-21-4 C7H6ClNO3 187.583 1-(环丙基甲氧基)-2-硝基苯 1-(cyclopropylmethoxy)-2-nitrobenzene 21315-09-3 C10H11NO3 193.202 —— 1-(sec-butoxy)-2-nitrobenzene 39645-92-6 C10H13NO3 195.218 2,4-二硝基苯甲醚 2,4-dinitroanisole 119-27-7 C7H6N2O5 198.135 4-溴-2-硝基苯甲醚 4-bromo-1-methoxy-2-nitrobenzene 33696-00-3 C7H6BrNO3 232.034 2-甲氧基-3-硝基苯酚 2-methoxy-3-nitrophenol 20734-71-8 C7H7NO4 169.137 4-碘-2-硝基苯甲醚 4-iodo-2-nitroanisole 52692-09-8 C7H6INO3 279.034 1-甲氧基-2-亚硝基苯 2-nitrosoanisole 17075-26-2 C7H7NO2 137.138 1-苄氧基-2-硝基苯 2-benzyloxynitrobenzene 4560-41-2 C13H11NO3 229.235 N-(2-甲氧基苯基)羟胺 N-(2-methoxyphenyl)hydroxylamine 35758-76-0 C7H9NO2 139.154 N-(3-甲氧基-4-硝基苯基)苯胺 3-methoxy-4-nitro-N-phenylaniline 723296-85-3 C13H12N2O3 244.25 3-甲氧基-4-硝基苯腈 3-methoxy-4-nitrobenzoinitrile 177476-75-4 C8H6N2O3 178.147 2-氟-6-硝基苯甲醚 1-fluoro-2-methoxy-3-nitrobenzene 484-94-6 C7H6FNO3 171.128 —— 4-((2-nitrophenoxy)methyl)tetrahydro-2H-pyran —— C12H15NO4 237.255 —— 1-methoxy-3-(113C)methyl-2-nitrobenzene 1347761-02-7 C8H9NO3 168.153 3-甲基-2-硝基苯甲醚 1-methoxy-3-methyl-2-nitro-benzene 5345-42-6 C8H9NO3 167.164 4-甲氧基-3-硝基苯甲基醇 (4-methoxy-3-nitrophenyl)methanol 41870-24-0 C8H9NO4 183.164 3-硝基-4-甲氧基氯苄 4-methoxy-3-nitrobenzyl chloride 6378-19-4 C8H8ClNO3 201.609 3-硝基-4-甲氧基苯甲醛 4-methoxy-3-nitrobenzaldehyde 31680-08-7 C8H7NO4 181.148 —— (3-methoxy-4-nitrophenyl)acetonitrile 104103-16-4 C9H8N2O3 192.174 1-甲氧基-4-甲氧基甲基-2-硝基-苯 1-methoxy-4-methoxymethyl-2-nitro-benzene 876494-02-9 C9H11NO4 197.191 —— 2-((2-nitrophenoxy)methyl)tetrahydrofuran 169330-16-9 C11H13NO4 223.229 1-甲氧基-4-[(4-甲氧基-3-硝基苯基)甲基]-2-硝基苯 bis-(4-methoxy-3-nitro-phenyl)-methane 6269-90-5 C15H14N2O6 318.286 —— 4-isopropyl-2-nitro-anisole 1706-61-2 C10H13NO3 195.218 —— 4-bromo-2,6-dinitroanisole 67856-76-2 C7H5BrN2O5 277.031 —— 2-Methoxy-4-(diphenylmethyl)nitrobenzene 189187-44-8 C20H17NO3 319.36 4-氯-2-硝基苯酚 p-chloro-o-nitrophenol 89-64-5 C6H4ClNO3 173.556 3-甲氧基-4-硝基苯乙酮 3-methoxy-4-nitroacetophenone 22106-39-4 C9H9NO4 195.175 —— 1-methoxy-2-nitro-4-phenoxymethyl-benzene —— C14H13NO4 259.262 —— 4-acetoxymethyl-1-methoxy-2-nitro-benzene —— C10H11NO5 225.201 —— 4-[(4-chlorophenyl)phenylmethyl]-2-methoxynitrobenzene —— C20H16ClNO3 353.805 4-甲氧基-3-硝基苯乙酮 1-(4-methoxy-3-nitrophenyl)ethanone 6277-38-9 C9H9NO4 195.175 1-甲氧基-3-(甲基-d)-2-硝基苯 1-(Deuteriomethyl)-3-methoxy-2-nitrobenzene 1304031-22-8 C8H9NO3 168.156 (3-甲氧基-4-硝基-苯基)-苯基-甲酮 3-methoxy-4-nitrobenzophenone 7501-57-7 C14H11NO4 257.246 —— 3-methoxy-4-nitropropiophenone —— C10H11NO4 209.202 2-(3-甲氧基-2-硝基苯基)乙腈 3-methoxy-2-nitrobenzeneacetonitrile 111795-89-2 C9H8N2O3 192.174 4-甲氧基-3-硝基苯磺酰氯 4-methoxy-3-nitrobenzene-1-sulfonyl chloride 22117-79-9 C7H6ClNO5S 251.647 —— 4-methoxy-3-nitro-benzenesulfonic acid 46403-72-9 C7H7NO6S 233.202 双(4-甲氧基-3-硝基苯基)甲酮 bis(4-methoxy-3-nitrophenyl)methanone 100881-20-7 C15H12N2O7 332.269 —— 4′-chloro-3-methoxy-4-nitrobenzophenone 213389-79-8 C14H10ClNO4 291.691 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:描述:2-硝基苯甲醚 在 盐酸 、 (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride 、 sodium periodate 、 copper(l) iodide 、 四(三苯基膦)钯 、 tin(II) chloride dihdyrate 、 乙醇 、 碘 、 potassium acetate 、 potassium carbonate 作用下, 以 乙醇 、 硫酸 、 二甲基亚砜 、 乙酸乙酯 、 甲苯 为溶剂, 反应 57.33h, 生成 4-(3-amino-4-methoxyphenyl)-5-methoxycoumarin hydrochloride参考文献:名称:4-芳基香豆素衍生的新型水溶性抗癌剂摘要:获得了新的 4-芳基香豆素衍生物。它们是众所周知的抗癌药物考布他汀 A-4 的水溶性类似物。合成的关键步骤涉及 Suzuki-Miyaura 交叉偶联。获得的水溶性盐对 HBL-100 和 HaCaT 细胞系表现出相当大的细胞毒活性。DOI:10.1007/s11172-013-0149-3
-
作为产物:描述:参考文献:名称:一种取代硝基苯类化合物的制备方法摘要:本发明公开了一种取代硝基苯类化合物的制备方法。该方法包括下述步骤:溶剂中,在150℃~250℃的温度下,化合物II在碱的作用下进行如下所示的脱羧反应得到化合物I即可;所述的碱为碱金属的碳酸盐和碳酸氢盐中的一种或多种。该方法相比一些使用金属催化的脱羧方法,具有操作简单、生产成本低、后处理方便、收率高的优点,在工业化生产中更具有应用价值。公开号:CN109467509B
-
作为试剂:描述:邻甲氧基苯胺 、 正丁醛 在 di-μ-chlorobis(norbornadiene)dirhodium(I) 、 2-硝基苯甲醚 作用下, 以 乙醇 为溶剂, 反应 4.0h, 以39%的产率得到3-乙基-8-甲氧基 -2-丙基-喹啉参考文献:名称:铑配合物催化氨基芳烃和脂肪醛合成喹啉摘要:在催化量的铑配合物和过量相应硝基芳烃的存在下,多种氨基芳烃在 180 °C 下与脂肪醛反应,以极好的收率得到 2-烷基和 2,3-二烷基取代的喹啉。在所检测的铑配合物中,[Rh(降冰片二烯)Cl]2 作为催化剂表现出最高的活性。因此,2-甲基-、2-乙基-3-甲基-、2-丙基-3-乙基-和2-丁基-3-丙基喹啉衍生物很容易从氨基芳烃和乙醛、丙醛、丁醛和戊醛中获得分别。DOI:10.1246/bcsj.54.3460
文献信息
-
Microwave-Assisted Rapid and efficient Reduction of Aromatic Nitro Compounds to Amines with Propan-2-ol over Nanosized Perovskite-type SmFeO<sub>3</sub> powder as a New Recyclable Heterogeneous Catalyst作者:Saeid Farhadi、Firouzeh Siadatnasab、Maryam KazemDOI:10.3184/174751911x12964930076647日期:2011.2
Nanosized perovskite-type SmFeO3 powder, prepared through the thermal decomposition of Sm[Fe(CN)6].4H2O with an average particle diameter of 28 nm and a specific surface area of 42 m2 g−1, was used as a recyclable heterogeneous catalyst for the efficient and selective reduction of aromatic nitro compounds into the corresponding amines by using propan-2-ol as a hydrogen donor (reducing agent) and KOH as a promoter under microwave irradiation. This highly regio- and chemoselective catalytic method is fast, clean, inexpensive, high yielding and also compatible with the substrates containing easily reducible functional groups. In addition, the nanosized SmFeO3 catalyst can be reused without loss of activity.
-
Cyclic (Alkyl)(amino)carbene Ligand-Promoted Nitro Deoxygenative Hydroboration with Chromium Catalysis: Scope, Mechanism, and Applications作者:Lixing Zhao、Chenyang Hu、Xuefeng Cong、Gongda Deng、Liu Leo Liu、Meiming Luo、Xiaoming ZengDOI:10.1021/jacs.0c12318日期:2021.1.27Transition metal catalysis that utilizes N-heterocyclic carbenes as noninnocent ligands in promoting transformations has not been well studied. We report here a cyclic (alkyl)(amino)carbene (CAAC) ligand-promoted nitro deoxygenative hydroboration with cost-effective chromium catalysis. Using 1 mol % of CAAC-Cr precatalyst, the addition of HBpin to nitro scaffolds leads to deoxygenation, allowing for利用 N-杂环卡宾作为非无害配体促进转化的过渡金属催化尚未得到很好的研究。我们在这里报告了具有成本效益的铬催化的环状(烷基)(氨基)卡宾(CAAC)配体促进的硝基脱氧硼氢化反应。使用 1 mol % 的 CAAC-Cr 预催化剂,将 HBpin 添加到硝基支架上会导致脱氧,从而保留各种可还原的官能团和敏感基团对硼氢化的相容性,从而提供一种温和、化学选择性和易于形成的策略苯胺,以及杂芳基和脂肪胺衍生物,具有广泛的范围和特别高的转换数(高达 1.8 × 106)。基于理论计算的机械研究,表明CAAC配体在促进HBpin氢化物极性反转中起重要作用;它用作 H 穿梭以促进脱氧硼氢化。通过这种策略制备的几种市售药物突出了其在药物化学中的潜在应用。
-
Enhanced catalytic performance of cobalt nanoparticles coated with a N,P-codoped carbon shell derived from biomass for transfer hydrogenation of functionalized nitroarenes作者:Yanan Duan、Tao Song、Xiaosu Dong、Yong YangDOI:10.1039/c8gc00619a日期:——(NPs) coated with a N,P-codoped carbon shell derived from naturally renewable biomass and earth-abundant, low-cost cobalt salt and PPh3. The entire process is operationally simple, straightforward, cost-effective and environmentally benign and can be used in mass production for practical application. The resultant catalysts allow for highly efficient and selective transfer hydrogenation of functionalized开发用于有机转化的大量可利用的贱金属催化剂仍然是化学研究的重要目标。在本文中,我们报道了第一种简便,快速,活性,廉价且可重复使用的钴纳米颗粒(NPs)的制备方法,该纳米颗粒涂有N,P掺杂的碳壳,该壳由天然可再生生物质和地球上丰富的低成本钴盐和PPh 3制成。。整个过程操作简单,直接,具有成本效益且对环境无害,可用于实际生产中的批量生产。所得的催化剂允许使用甲酸或甲酸铵作为氢供体将官能化的硝基芳烃高效且选择性地转移氢化成相应的苯胺。均匀掺入碳晶格中的N和P与包封的Co NPs表现出协同效应,以工程化催化剂的结构和组成,从而大大提高了催化效率。最具活性的催化剂Co @ NPC-800表现出出色的活性和选择性,可将官能化的硝基芳烃还原为苯胺,特别是装饰有易于还原的官能团的苯胺。
-
Base-free chemoselective transfer hydrogenation of nitroarenes to anilines with formic acid as hydrogen source by a reusable heterogeneous Pd/ZrP catalyst作者:Jaya Tuteja、Shun Nishimura、Kohki EbitaniDOI:10.1039/c4ra06174h日期:——transfer hydrogenation (CTH) of nitroarenes using FA as a hydrogen source. Various supported Pd catalysts were examined for this transformation, and Pd supported ZrP (Pd/ZrP) proved to be the best catalyst for CTH of nitrobenzene. Applicability of the Pd/ZrP catalyst is also explored for hydrogenation of various substituted nitroarenes. The Pd/ZrP catalyst showed high specificity for hydrogenation of nitro开发了一种高效的,化学选择性的,环境友好的方法,该方法使用FA作为氢源,对硝基芳烃进行催化转移氢化(CTH)。研究了各种负载的Pd催化剂的这种转化,Pd负载的ZrP(Pd / ZrP)被证明是硝基苯CTH的最佳催化剂。还探讨了Pd / ZrP催化剂在各种取代硝基芳烃氢化中的适用性。Pd / ZrP催化剂即使在其他可还原的官能团(例如–C C,–COOCH 3和–C N)存在下,也表现出对硝基氢化的高特异性。为研究反应机理,获得了CTH的Hammett图对取代的硝基芳烃。活动站点被认为是在原地从XRD和TEM数据可以看出,生成了Pd(0)物质。Pd / ZrP催化剂可重复使用至少4次,同时保持相同的活性和选择性。据我们所知,这是在无碱条件下对硝基芳烃进行CTH的最佳方法之一,与多相Pd基催化剂相比,该方法具有高活性和化学选择性。
-
Green synthesis and catalytic properties of palladium nanoparticles for the direct reductive amination of aldehydes and hydrogenation of unsaturated ketones作者:Mahmoud NasrollahzadehDOI:10.1039/c4nj01440e日期:——This paper reports on the synthesis and use of palladium nanoparticles as heterogeneous catalysts for the reductive amination of aldehydes and hydrogenation of unsaturated ketones. This method has the advantages of high yields, simple methodology and easy work up. The catalyst can be recovered and reused several times without significant loss of catalytic activity.本文报道了钯纳米粒子的合成及其作为非均相催化剂用于醛的还原胺化和不饱和酮加氢的应用。该方法具有收率高,方法简单,后处理容易的优点。催化剂可以回收并重复使用几次,而不会显着降低催化活性。
表征谱图
-
氢谱1HNMR
-
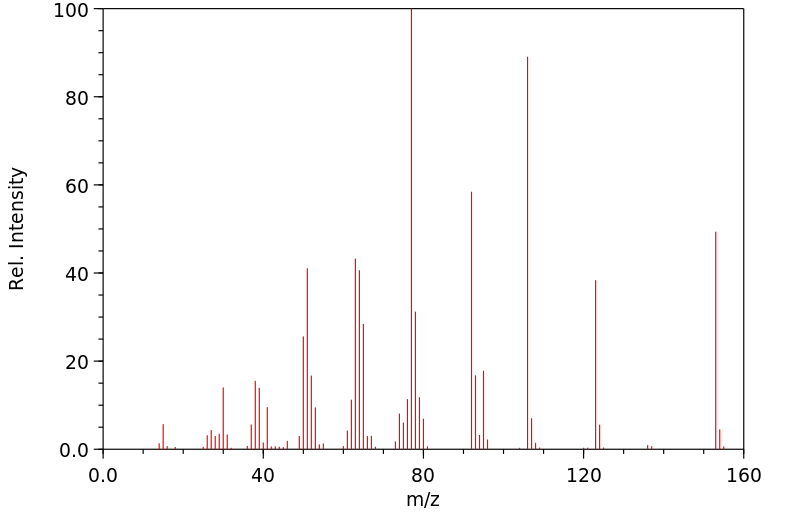
质谱MS
-
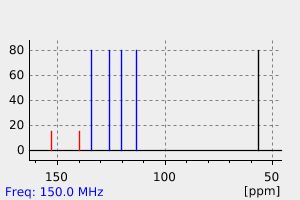
碳谱13CNMR
-
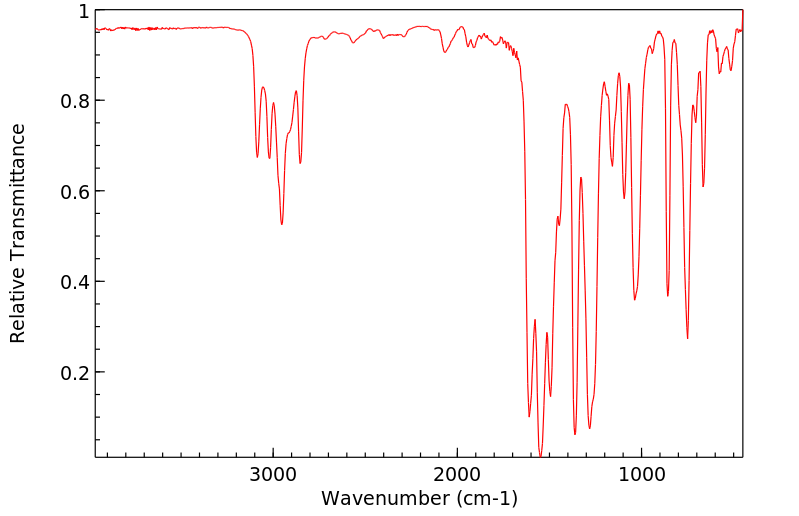
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息