己二酸,聚合2,2-二甲基-1,3-丙二醇和2,5-呋喃二酮,苯酸酯 | 9004-78-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:11-13 °C (lit.)
-
沸点:247 °C (lit.)
-
密度:1.102 g/mL at 25 °C (lit.)
-
蒸气密度:4.8 (vs air)
-
闪点:>230 °F
-
溶解度:可溶、透明、无色至微黄色
-
LogP:1.2 at 23℃
-
物理描述:Ethylene glycol phenyl ether is a colorless liquid with a pleasant odor. Density 1.02 g / cm3. An irritant.
-
颜色/状态:Oily liquid
-
气味:Faint aromatic odor
-
味道:Burning taste
-
蒸汽密度:4.77 (NTP, 1992) (Relative to Air)
-
蒸汽压力:0.007 mm Hg at 25 °C
-
稳定性/保质期:
STABLE IN PRESENCE OF ACIDS & ALKALIES.
-
自燃温度:500 °C
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
粘度:20.5 centistokes at 25 °C
-
燃烧热:958 kcal/mole
-
表面张力:42.0 dynes/cm
-
折光率:Index of refraction: 1.534 at 20 °C/D
-
解离常数:pKa = 15.10 at 25 °C
-
相对蒸发率:< 0.01 (Butyl acetate = 1.0)
-
保留指数:1191.6;1186;1210;1214;1213;1204.2;1187.1;1189;1185;1194
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:10
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
制备方法与用途
传统防腐剂
苯氧乙醇(Phenoxyethanol)是一种传统防腐剂,为无色稍带黏性的油状液体,具有轻微的芳香气味和火辣味。它微溶于水,基本不溶于矿物油,可与丙酮、乙醇和甘油任意混合。
苯氧乙醇对铜绿假单胞菌(绿脓杆菌)有显著杀灭效果,并能抑制革兰阳性菌及革兰阴性菌的生长。其机制是通过作用于细胞膜增大钾离子的透过率,降低酶活性,从而抑制细胞生长达到抗菌效果。单独使用苯氧乙醇时,低浓度下防腐效果不佳,高浓度又可能引起皮肤刺痛和灼热感,因此需要与其他防腐成分复配使用。
毒性
作为当前防腐剂标准的化合物之一,苯氧乙醇一般不会释放甲醛,相较于会释放甲醛的其他化合物,它是一个更优秀的替代品。根据《化妆品防腐剂的现状与发展》2007版数据显示,苯氧乙醇的大鼠经口半数致死量为3000 mg/kg,小鼠为4000 mg/kg,属于轻度毒性。
合成方法
采用苯酚一环氧乙烷法制备苯氧乙醇:在乙酸钠或氢氧化钠的作用下,苯酚与环氧乙烷缩合。反应后通过粗品减压蒸馏得成品,该工艺中苯酚转化率≥99%,合成收率≥95%,总产品收率达90%。反应方程式如下:
[ \text{Phenol} + \text{EO} \xrightarrow{\text{NaAc/H}_2\text{O}} \text{Phenoxyethanol} ]
此方法需在温度20 ℃和0.2―0.25 MPa的压力下进行,适合大规模工业生产。
化学性质
苯氧乙醇为无色液状液体。熔点14℃,沸点245.2℃。易溶于醇、醚及氢氧化钠溶液,微溶于水,在酸或碱中稳定,并具有芳香气味和烧灼味。
用途
苯氧乙醇是一种高沸点有机溶剂,可用于水性涂料及油墨成膜助剂、香料定香剂、油墨流畅剂、药用防腐杀菌剂、电子清洗剂及油墨慢干剂等。同时,它也具有良好的溶解性能,能与多种树脂如丙烯酸树脂、硝基纤维素、乙基纤维素、环氧树脂、醇酸树脂和苯氧基树脂等多种有机物混溶。
生产方法
由苯酚和环氧乙烷加成而得,在乙酸钠或氢氧化钠存在下反应。反应温度为200℃,压力为0.2-0.25 MPa。
参考资料
- 周湘云,刘称心. 苯氧乙醇的性质、制备和应用[J]. 化工时刊, 1999(05):24-26.
- 张亨. 苯氧乙醇的合成研究进展[J]. 化工中间体, 2013, 10(10):13-19.
- 杨杰,李程碑. 乙基己基甘油与苯氧乙醇复配体的研究[J]. 广东化工, 2016, 43(02):55-56+58.
- 廖子逸. 化妆品防腐保湿剂的应用进展及发展趋势[J]. 山东化工, 2018, 47(05):77-78+82.
- 郭阳,臧埔,郜玉钢等. 化妆品防腐剂的使用现状及进展[J]. 中南药学, 2018, 16(09):1258-1263.
- 杨娟,刘永龙,林芮等. 几种常用防腐剂对日化产品中腐败微生物抑制效果研究[J]. 工业微生物, 2016, 46(04):34-37.
- 李欣航,张金龙,毕永贤,等. 无尼泊金酯类化妆品防腐体系的应用研究[J]. 日用化学品科学, 2018, 41(8):14-21.
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基乙氧基苯 2-methoxyethyl phenyl ether 41532-81-4 C9H12O2 152.193 苯氧乙酸 2-phenoxyacetic acid 122-59-8 C8H8O3 152.15 烯丙基苯基醚 allyl phenyl ether 1746-13-0 C9H10O 134.178 乙二醇苯醚醋酸酯 2-phenoxyethyl acetate 6192-44-5 C10H12O3 180.203 苯氧乙酸甲酯 methyl 2-phenoxyacetate 2065-23-8 C9H10O3 166.177 —— 2-phenoxyethanol, TMS derivative 16654-47-0 C11H18O2Si 210.348 1-苯氧乙醇 phenoxyethyl alcohol 56101-99-6 C8H10O2 138.166 苯氧乙酰氯 Phenoxyacetyl chloride 701-99-5 C8H7ClO2 170.595 —— bis(2-phenoxyethyl)carbonate 22855-36-3 C17H18O5 302.327 苯氧乙酸乙酯 phenoxyacetic acid ethyl ester 2555-49-9 C10H12O3 180.203 对氯苯氧乙酸 4-Chlorophenoxyacetic acid 122-88-3 C8H7ClO3 186.595 —— [(2-phenoxyethoxy)methyl]benzene 84877-70-3 C15H16O2 228.291 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2-二苯氧基乙烷 1,2-diphenoxyethane 104-66-5 C14H14O2 214.264 2-甲氧基乙氧基苯 2-methoxyethyl phenyl ether 41532-81-4 C9H12O2 152.193 苯酚,4-(2-羟基乙氧基)- 4-(2-hydroxyethoxy)-phenol 13427-53-7 C8H10O3 154.166 O-[2-(苯氧基)乙基]羟胺 O-(2-phenoxyethyl)hydroxylamine 73941-29-4 C8H11NO2 153.181 苯乙醚 Phenetole 103-73-1 C8H10O 122.167 —— 1-ethyl-4-phenylethylene glycol 19594-02-6 C10H14O2 166.22 2-苯氧基乙醇甲酸酯 2-phenoxyethyl formate 58214-97-4 C9H10O3 166.177 —— 1,5-diphenoxy-3-oxapentane 622-87-7 C16H18O3 258.317 —— 2-phenoxyethyl vinyl ether 18370-86-0 C10H12O2 164.204 —— 2-phenoxyethoxymethyl chloride 102331-29-3 C9H11ClO2 186.638 苯氧乙酸 2-phenoxyacetic acid 122-59-8 C8H8O3 152.15 (2-碘乙氧基)苯 (2-iodoethoxy)benzene 37137-00-1 C8H9IO 248.063 —— (2-(methoxymethoxy)ethoxy)benzene 90926-61-7 C10H14O3 182.219 苯氧代乙醛 2-phenoxyethanal 2120-70-9 C8H8O2 136.15 2-氟乙氧基苯 (2-fluoroethoxy)benzene 405-97-0 C8H9FO 140.157 2-氯苯乙醚 2-chloroethoxybenzene 622-86-6 C8H9ClO 156.612 2-苯氧基乙硫醇 2-Phenoxy-aethylmercaptan 6338-63-2 C8H10OS 154.233 —— O-(2-hydroxyethyl)resorcinol 49650-88-6 C8H10O3 154.166 —— 1-allyloxy-2-phenoxy-ethane 93066-80-9 C11H14O2 178.231 2-苯氧乙基溴 phenoxyethyl bromide 589-10-6 C8H9BrO 201.063 1,1'-[亚甲基二(氧基乙烷-1,2-二基氧基)]二苯 bis-[(2-phenoxyethyl)oxy]methane 13879-32-8 C17H20O4 288.343 2-苯氧基乙胺 2-phenoxyethanamine 1758-46-9 C8H11NO 137.181 2-(4-氯苯氧)乙醇 2-(4-chlorophenoxy)ethanol 1892-43-9 C8H9ClO2 172.611 2-(4-碘-苯氧基)-乙醇 2-(4-iodophenoxy)ethanol 29639-77-8 C8H9IO2 264.063 2-(4-氨基苯氧基)乙醇 2-(4-aminophenoxy)ethanol 6421-88-1 C8H11NO2 153.181 2-(4-溴苯氧基)乙醇 2-(4-bromophenoxy)ethanol 34743-88-9 C8H9BrO2 217.062 3-(2-苯氧基乙氧基)丙腈 3-(2-phenoxy-ethoxy)-propionitrile 6328-54-7 C11H13NO2 191.23 乙二醇苯醚醋酸酯 2-phenoxyethyl acetate 6192-44-5 C10H12O3 180.203 2-苯氧基乙基氯甲酸酯 1-chlorocarbonyloxy-2-phenoxy-ethane 34743-87-8 C9H9ClO3 200.622 —— 2-phenoxyethyl carbamate 31432-77-6 C9H11NO3 181.191 —— 2-phenoxyethanol, TMS derivative 16654-47-0 C11H18O2Si 210.348 —— 4-phenoxy-1-butyn-3-ol 88462-64-0 C10H10O2 162.188 N-甲基-2-苯氧基乙基胺 N-methyl-2-phenoxyethanamine 37421-04-8 C9H13NO 151.208 2-(2-羟基乙氧基)苯酚 2-(2-hydroxyphenoxy)ethanol 4792-78-3 C8H10O3 154.166 苯甲醚 methoxybenzene 100-66-3 C7H8O 108.14 —— Phosphorigsaeure-di-<2-phenoxy-ethylester> —— C16H19O5P 322.298 —— 3-(2- ethoxy)-propan-1,2-diol 170678-39-4 C11H16O4 212.246 —— 2-phenoxyethyl methylcarbonate —— C10H12O4 196.203 三(2-苯氧基乙基)亚磷酸酯 Tris-<2-phenoxy-ethyl>-phosphit 4486-47-9 C24H27O6P 442.449 —— (E)-1,4-diphenoxybut-2-ene 15779-52-9 C16H16O2 240.302 —— 1-phenoxyethyl-3-methoxy-1,2-propanediol 153904-87-1 C12H18O4 226.273 丙酸-2-苯氧基乙酯 2-phenoxyethyl propanoate 23495-12-7 C11H14O3 194.23 —— carbonic acid ethyl ester-(2-phenoxy-ethyl ester) 40496-06-8 C11H14O4 210.23 —— bis(2-phenoxyethyl)carbonate 22855-36-3 C17H18O5 302.327 —— ethylene glycol monophenyl ether phosphate 18168-30-4 C8H11O5P 218.146 N,N-二甲基-2-苯氧基乙胺 dimethyl(2-phenoxyethyl)amine 13468-02-5 C10H15NO 165.235 2-叠氮乙氧基苯 2-(phenoxy)ethyl azide 70659-90-4 C8H9N3O 163.179 2-苯氧基乙基氯乙酸酯 2-phenoxyethyl chloroacetate 103-57-1 C10H11ClO3 214.649 —— 2-<(2-phenoxyethoxy)methyl>oxirane 14435-46-2 C11H14O3 194.23 1,4-苯并二恶烷 benzo-1,4-dioxane 493-09-4 C8H8O2 136.15 2-苯氧基乙基单溴乙酸酯 2-bromoacetic acid 2-phenoxyethyl ester 56521-82-5 C10H11BrO3 259.1 2-苯氧基乙基丙烯酸酯 2-phenoxyethyl acrylate 48145-04-6 C11H12O3 192.214 —— 1-Chloro-3-(2-phenoxyethoxy)propan-2-OL 55773-75-6 C11H15ClO3 230.691 对氯苯氧乙酸 4-Chlorophenoxyacetic acid 122-88-3 C8H7ClO3 186.595 —— sulfuric acid mono-(2-phenoxy-ethyl ester) 37930-24-8 C8H10O5S 218.23 —— 2-phenoxyethyl methanesulfonate 141482-06-6 C9H12O4S 216.258 —— methoxy-acetic acid-(2-phenoxy-ethyl ester) 60359-62-8 C11H14O4 210.23 —— (2-phenoxy-ethoxy)-acetic acid ethyl ester 2296-06-2 C12H16O4 224.257 —— β-phenoxyethyl 3-hydroxy propionate 60359-41-3 C11H14O4 210.23 [2-(2-苯氧基乙氧基)乙基]二乙胺 5275525 (Hit2Lead) 24480-59-9 C14H23NO2 237.342 —— tri(ethoxy)(2-phenoxyethoxy)silane —— C14H24O5Si 300.427 —— di(ethoxy)di(2-phenoxyethoxy)silane —— C20H28O6Si 392.524 —— di(methoxy)di(2-phenoxyethoxy)silane —— C18H24O6Si 364.47 —— Si(Oet-2-Oph)4 18758-29-7 C32H36O8Si 576.719 —— phenoxyacetic acid 2-phenoxyethyl ester 5421-29-4 C16H16O4 272.301 —— 1-phenoxy-2-p-ethylphenoxyethane 107550-63-0 C16H18O2 242.318 2-苯氧乙基丁酸酯 2-phenoxyethyl butyrate 23511-70-8 C12H16O3 208.257 —— Cyanessigsaeure-β-phenoxyethyl-ester 32804-78-7 C11H11NO3 205.213 —— 3-methoxy-propionic acid-(2-phenoxy-ethyl ester) —— C12H16O4 224.257 2-(2-氨基苯氧基)乙醇 2-(2-aminophenoxy)ethanol 42876-07-3 C8H11NO2 153.181 1-溴-4-(2-丁氧基乙氧基)苯 1-bromo-4-[2-(butoxy)ethoxy]benzene 39255-24-8 C12H17BrO2 273.17 2-苯氧基乙基丁-2-烯酸酯 2-Phenoxyethyl but-2-enoate 60359-25-3 C12H14O3 206.241 —— oxydi-acetic acid bis-(2-phenoxy-ethyl ester) 16529-54-7 C20H22O7 374.39 苯基乙烯醚 vinyl phenyl ether 766-94-9 C8H8O 120.151 2-(2-溴-苯氧基)-乙醇 2-(2-bromophenoxy)ethanol 34743-89-0 C8H9BrO2 217.062 —— 1-(2-phenoxy-ethoxy)-1,2,2-trifluoroethene 245108-08-1 C10H9F3O2 218.175 —— 2-Phenoxyethyl [(propan-2-yl)oxy]acetate 60359-63-9 C13H18O4 238.284 —— 2-phenoxyethyl pivalate 58214-99-6 C13H18O3 222.284 —— β-phenoxyethyl O-acetylglycolate 60359-58-2 C12H14O5 238.24 1-(异丙基氨基)-3-苯氧基-2-丙醇 (+/-)-1-isopropylamino-3-phenoxypropan-2-ol 7695-63-8 C12H19NO2 209.288 —— 1-(butylamino)-3-phenoxypropan-2-ol 29638-63-9 C13H21NO2 223.315 —— DL-lactic acid-(2-phenoxy-ethyl ester) 6283-84-7 C11H14O4 210.23 —— 1-phenoxybut-3-yn-2-one 126959-19-1 C10H8O2 160.172 —— phosphoric acid bis-(2-phenoxy-ethyl ester) 107433-91-0 C16H19O6P 338.297 —— 2-Phenoxy-aethyl-trimethylsilylsulfid 16654-62-9 C11H18OSSi 226.415 三乙基(2-苯氧基乙氧基)硅烷 2-Phenoxy-aethyl-triaethylsilylaether 16654-60-7 C14H24O2Si 252.429 —— [(2-phenoxyethoxy)methyl]benzene 84877-70-3 C15H16O2 228.291 —— β-phenoxyethyl pyruvate 60359-42-4 C11H12O4 208.214 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
反应信息
-
作为反应物:描述:己二酸,聚合2,2-二甲基-1,3-丙二醇和2,5-呋喃二酮,苯酸酯 在 氧气 、 copper(l) chloride 作用下, 以 二甲基亚砜 为溶剂, 120.0 ℃ 、101.33 kPa 条件下, 反应 6.0h, 以74%的产率得到对苯醌参考文献:名称:通过连续的C–C键裂解 将羟基化合物逐步降解为醛†摘要:通过使用不带任何配体和添加剂的双金属催化体系(PdCl 2 + CuCl),通过C-C键的连续裂解将羟基化合物逐步降解为醛。广泛的适用性扩展到芳族,脂肪族,伯和仲醇以及木质素模型化合物的各种范围。DOI:10.1039/c8cc09504c
-
作为产物:参考文献:名称:利用钛催化更安全地还原羧酸摘要:先前显示氨硼烷在回流下与羧酸反应形成伯酰胺,在催化 TiCl 4存在下在室温下将酸还原为醇。该方法能够耐受多种潜在反应性官能团,包括N-保护的氨基酸,可用于在酰胺、腈以及某种程度上酯存在的情况下选择性还原酸。在芳香酸存在下,可以选择性地还原脂肪酸。DOI:10.1021/acs.orglett.2c03326
-
作为试剂:参考文献:名称:[EN] PROCESS FOR PREPARING DTEA HCI
[FR] PROCÉDÉ DE PRÉPARATION DE DTHEA HCI摘要:本发明提供了一种改进的方法,用于通过使用催化剂、溶剂和辅助反应的共溶剂,从癸烯和半胱氨酸盐酸盐制备DTEA HCl,并提供所得产物溶液的低温稳定化。公开号:WO2021216029A1
文献信息
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
Quinolone-based compounds, formulations, and uses thereof申请人:Manetsch Roman公开号:US10000452B1公开(公告)日:2018-06-19Provided herein are quinolone-based compounds that can be used for treatment and/or prevention of malaria and formulations thereof. Also provided herein are methods of treating and/or preventing malaria in a subject by administering a quinolone-based compound or formulation thereof provided herein.
-
PHOTOSENSITIVE RESIN COMPOSITION, OXIME SULFONATE COMPOUND, METHOD FOR FORMING CURED FILM, CURED FILM, ORGANIC EL DISPLAY DEVICE, AND LIQUID CRYSTAL DISPLAY DEVICE申请人:FUJIFILM Corporation公开号:US20130171415A1公开(公告)日:2013-07-04Disclosed is a photosensitive resin composition comprising: (Component A) an oxime sulfonate compound represented by Formula (1); (Component B) a resin comprising a constituent unit having an acid-decomposable group that is decomposed by an acid to form a carboxyl group or a phenolic hydroxy group; and (Component C) a solvent wherein in Formula (1) R 1 denotes an alkyl group, an aryl group, or a heteroaryl group, each R 2 independently denotes a hydrogen atom, an alkyl group, an aryl group, or a halogen atom, Ar 1 denotes an o-arylene group or an o-heteroarylene group, X denotes O or S, and n denotes 1 or 2, provided that of two or more R 2 s present in the compound, at least one denotes an alkyl group, an aryl group, or a halogen atom.
-
[EN] PYRIMIDINE JAK INHIBITORS FOR THE TREATMENT OF SKIN DISEASES<br/>[FR] INHIBITEURS DE JAK À BASE DE PYRIMIDINE POUR LE TRAITEMENT DE MALADIES DE LA PEAU申请人:THERAVANCE BIOPHARMA R&D IP LLC公开号:WO2020219640A1公开(公告)日:2020-10-29The invention provides compounds of formula (I): or pharmaceutically-acceptable salts thereof, that are inhibitors of Janus kinases. The invention also provides pharmaceutical compositions comprising such compounds, and methods of using such compounds to treat inflammatory and autoimmune skin diseases.该发明提供了式(I)的化合物或其药用可接受盐,这些化合物是Janus激酶的抑制剂。该发明还提供了包含这些化合物的药物组合物,以及使用这些化合物治疗炎症性和自身免疫性皮肤疾病的方法。
-
BRM TARGETING COMPOUNDS AND ASSOCIATED METHODS OF USE申请人:Arvinas Operations, Inc.公开号:US20190300521A1公开(公告)日:2019-10-03The present disclosure relates to bifunctional compounds, which find utility as modulators of SMARCA2 or BRM (target protein). In particular, the present disclosure is directed to bifunctional compounds, which contain on one end a ligand that binds to the Von Hippel-Lindau E3 ubiquitin ligase, and on the other end a moiety which binds the target protein, such that the target protein is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of target protein. The present disclosure exhibits a broad range of pharmacological activities associated with degradation/inhibition of target protein. Diseases or disorders that result from aggregation or accumulation of the target protein are treated or prevented with compounds and compositions of the present disclosure.本公开涉及双功能化合物,其作为SMARCA2或BRM(靶蛋白)的调节剂具有实用性。具体而言,本公开涉及包含一端结合Von Hippel-Lindau E3泛素连接酶的配体,另一端结合靶蛋白的双功能化合物,使得靶蛋白与泛素连接酶靠近以实现靶蛋白的降解(和抑制)。本公开展示了与靶蛋白降解/抑制相关的广泛药理活性。本公开的化合物和组合物用于治疗或预防由靶蛋白聚集或积累导致的疾病或紊乱。
表征谱图
-
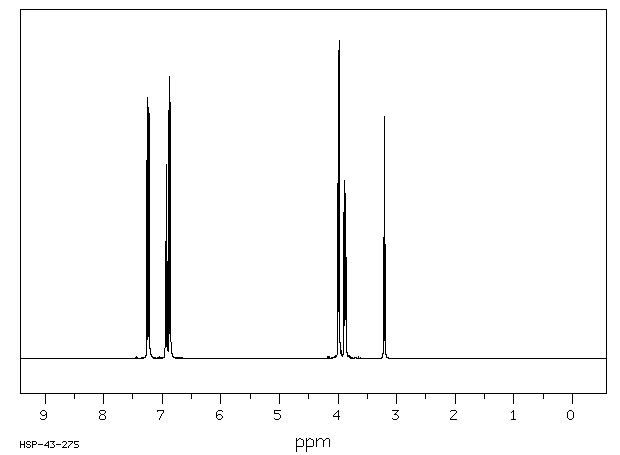
氢谱1HNMR
-
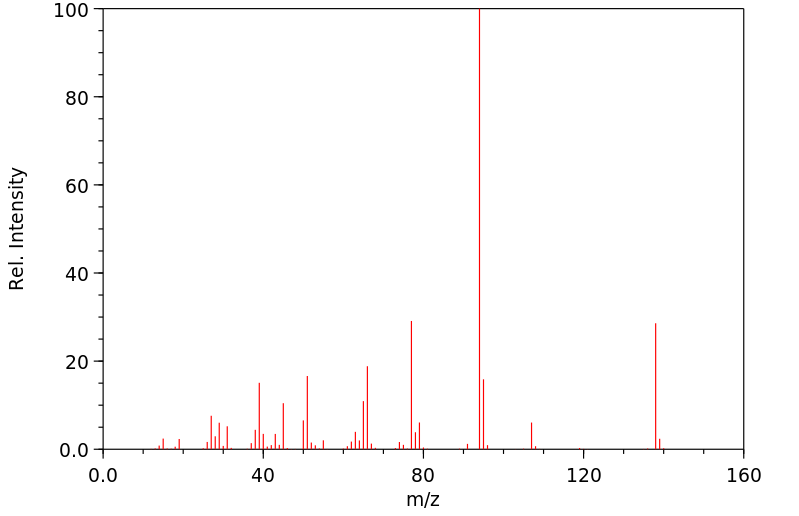
质谱MS
-
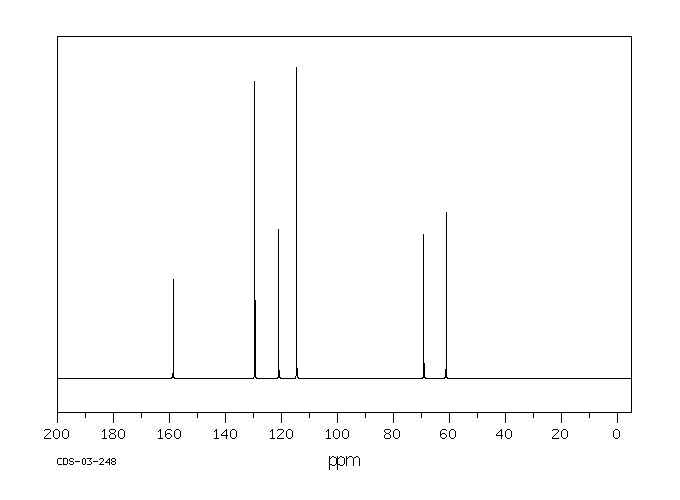
碳谱13CNMR
-
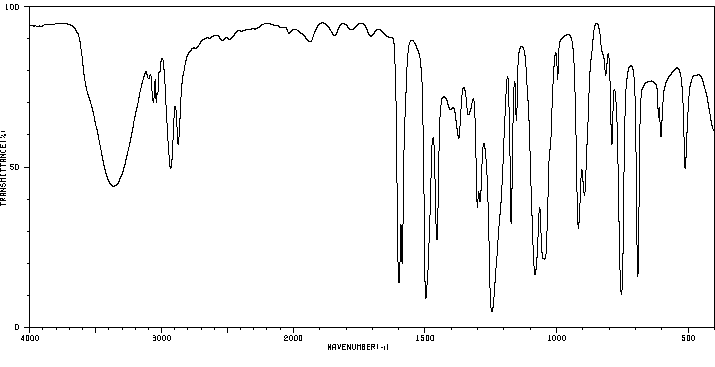
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息