间苯胺氢氟乙酰氯 | 27191-09-9
物质功能分类
中文名称
间苯胺氢氟乙酰氯
中文别名
间茴香胺盐酸盐;间氨基苯甲醚盐酸盐;3-甲氧基苯胺盐酸盐;M-氨基苯甲醚盐酸盐
英文名称
m-anisidine hydrochloride
英文别名
3-methoxyaniline hydrochloride;3-methoxyaniline;hydrochloride
CAS
27191-09-9
化学式
C7H10NO*Cl
mdl
MFCD00060220
分子量
159.615
InChiKey
FNCKZWCTEPKMQV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172.0 to 176.0 °C
计算性质
-
辛醇/水分配系数(LogP):0.99
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:35.2
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险等级:6.1
-
海关编码:2922299090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温且干燥
SDS
间茴香胺盐酸盐 修改号码:5
模块 1. 化学品
产品名称: m-Anisidine Hydrochloride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
急性毒性(经皮) 第3级
急性毒性(吸入) 第3级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
特异性靶器官毒性 血液
- 单一接触 [第2级]
特异性靶器官毒性 血液
- 单一接触 [第1级]
环境危害
急性水生毒性 第1级
慢性水生毒性 第1级
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吸入或皮肤接触或吞咽会中毒。
造成皮肤刺激
造成严重眼刺激
可能对器官产生损害: 血液
可能因延长或接触对器官产生损害: 血液
对水生生物有极毒性
长期影响对水生生物有极毒性
防范说明
间茴香胺盐酸盐 修改号码:5
模块 2. 危险性概述
[预防] 切勿吸入。
只能在室外或通风良好的环境下使用。
避免释放到环境中。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。呼叫解毒中心/医生。
食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
立即去除/脱掉所有被污染的衣物。
被污染的衣物清洗后方可重新使用。
如接触到或感不适:呼叫解毒中心/医生。
收集溢出物。
[储存] 存放于通风良好处。保持容器密闭。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 间茴香胺盐酸盐
百分比: >98.0%(LC)(N)
CAS编码: 27191-09-9
俗名: 3-Methoxyaniline Hydrochloride
分子式: C7H9NO·HCl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
间茴香胺盐酸盐 修改号码:5
模块 6. 泄漏应急处理
环保措施: 小心,切勿排入河流等。因为考虑对环境有负面影响。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅红黄色
气味: 无资料
pH: 无数据资料
熔点:
174°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氯化氢
间茴香胺盐酸盐 修改号码:5
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 2431
正式运输名称: 甲氧基苯胺
包装等级: III
海洋污染物: Y
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: m-Anisidine Hydrochloride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
急性毒性(经皮) 第3级
急性毒性(吸入) 第3级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
特异性靶器官毒性 血液
- 单一接触 [第2级]
特异性靶器官毒性 血液
- 单一接触 [第1级]
环境危害
急性水生毒性 第1级
慢性水生毒性 第1级
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吸入或皮肤接触或吞咽会中毒。
造成皮肤刺激
造成严重眼刺激
可能对器官产生损害: 血液
可能因延长或接触对器官产生损害: 血液
对水生生物有极毒性
长期影响对水生生物有极毒性
防范说明
间茴香胺盐酸盐 修改号码:5
模块 2. 危险性概述
[预防] 切勿吸入。
只能在室外或通风良好的环境下使用。
避免释放到环境中。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。呼叫解毒中心/医生。
食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
立即去除/脱掉所有被污染的衣物。
被污染的衣物清洗后方可重新使用。
如接触到或感不适:呼叫解毒中心/医生。
收集溢出物。
[储存] 存放于通风良好处。保持容器密闭。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 间茴香胺盐酸盐
百分比: >98.0%(LC)(N)
CAS编码: 27191-09-9
俗名: 3-Methoxyaniline Hydrochloride
分子式: C7H9NO·HCl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
间茴香胺盐酸盐 修改号码:5
模块 6. 泄漏应急处理
环保措施: 小心,切勿排入河流等。因为考虑对环境有负面影响。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅红黄色
气味: 无资料
pH: 无数据资料
熔点:
174°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氯化氢
间茴香胺盐酸盐 修改号码:5
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 2431
正式运输名称: 甲氧基苯胺
包装等级: III
海洋污染物: Y
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis and Characterization of 3-Arylquinazolinone and 3-Arylquinazolinethione Derivatives as Selective Estrogen Receptor Beta Modulators摘要:On the basis of the stucture of genistein, a new series of 3-arylquinazolines was prepared and tested for their estrogen receptor (ER) alpha and beta affinities. 5,7-Dihydroxy-3 -(4-hydroxyphenyl)-4(3H)-quinazolinone (1aa) acts as an agonist on both ER subtypes. It has 62-fold higher binding affinity [IC50(ER beta) = 179 nM] and 38-fold higher functional potency in a transcription assay [EC50(ER beta) = 76 nM] with ER beta than with ER alpha, thus improving upon the selectivity of genistein. All of the analogues showed preferential binding affinity for ER. Many are also more potent in activating transcription by ER beta than by ER alpha. Transformation of the C=O functionality at position 4 into a C=S group provided 5,7-dihydroxy-3-(4-hydroxyphenyl)4(3H)-quinazolinethione (1ba), which acts as an agonist on both ER subtypes but has 56-fold higher binding affinity for ER beta over ER alpha [IC50(ER beta) = 47 nM] and 215-fold higher potency in the transcription assay [EC50(ER beta) = 13 nM]. These ER beta-selective compounds may represent valuable tools in understanding the differences in structure and biological function of ER beta and ER alpha.DOI:10.1021/jm0509389
-
作为产物:参考文献:名称:Iron-catalysed, general and operationally simple formal hydrogenation using Fe(OTf)3 and NaBH4摘要:已开发出一种操作简单且环境友好的正规氢化方案,使用高丰度的铁(III)盐和廉价、台面稳定、化学计量还原剂NaBH4,在乙醇中,在常温下进行。DOI:10.1039/c4ob00945b
-
作为试剂:参考文献:名称:Matlin, Stephen A.; Barron, Kenneth, Journal of Chemical Research, Miniprint, 1990, # 8, p. 1920 - 1943摘要:DOI:
文献信息
-
Synthesis of Primary Amines by the Electrophilic Amination of Grignard Reagents with 1,3-Dioxolan-2-one <i>O</i>-Sulfonyloxime作者:Mitsuru Kitamura、Takahiro Suga、Shunsuke Chiba、Koichi NarasakaDOI:10.1021/ol0479951日期:2004.11.1Primary amines are prepared by the electrophilic amination of Grignard reagents with 4,4,5,5-tetramethyl-1,3-dioxolan-2-one O-phenylsulfonyloxime and the acidic hydrolysis of the resulting imines. [reaction: see text]
-
[EN] TRICYCLIC AZAINDOLES<br/>[FR] AZAINDOLES TRICYCLIQUES申请人:MERCK PATENT GMBH公开号:WO2010080253A1公开(公告)日:2010-07-15Disclosed are dipyridyl-pyrrole derivative compounds and analogs thereof, pharmaceutical compositions comprising such compounds and processes for preparing the same. The compounds are useful in the treatment of diseases amenable to protein kinase signal transduction inhibition, regulation and/or modulation.
-
QSAR modeling of synthesized 3-(1,3-benzothiazol-2-yl) 2-phenyl quinazolin-4(3H)-ones as potent antibacterial agents作者:Pratibha Sharma、Ashok Kumar、Prerna Kumari、Jitendra Singh、M. P. KaushikDOI:10.1007/s00044-011-9626-0日期:2012.7on the basis of IR, 1H NMR, Mass, and elemental analysis data. These compounds were screened in vitro for their antibacterial activity against a representative panel of Gram positive and Gram negative bacteria and models were generated through quantitative structure–activity relationship (QSAR).The activity contributions due to structural and substituent effects were determined using sequential regression
-
Enantioselective Deaminative Alkylation of Amino Acid Derivatives with Unactivated Olefins作者:Shang-Zheng Sun、Yue-Ming Cai、De-Liang Zhang、Jia-Bao Wang、Hong-Qing Yao、Xi-Yan Rui、Ruben Martin、Ming ShangDOI:10.1021/jacs.1c12350日期:2022.1.26sterically encumbered bis(oxazoline) ligand backbone, thus offering a de novo technology for accessing enantioenriched sp3–sp3 linkages via sp3 C–N functionalization. Our protocol is distinguished by its broad scope and generality across a wide number of counterparts, even in the context of late-stage functionalization. In addition, an enantioselective deaminative remote hydroalkylation reaction of unactivated
-
The Ortho Effect on the Acidic and Alkaline Hydrolysis of Substituted Formanilides作者:Salil Dileep Desai、Lee E. KirschDOI:10.1002/kin.20925日期:2015.8kinetics of formanilides hydrolysis were determined under first‐order conditions in hydrochloric acid (0.01–8 M, 20–60°C) and in hydroxide solutions (0.01–3 M, 25 and 40°C). Under acidic conditions, second‐order specific acid catalytic constants were used to construct Hammett plots. The ortho effect was analyzed using the Fujita–Nishioka method. In alkaline solutions, hydrolysis displayed both first‐在一级条件下,在盐酸(0.01–8 M,20–60°C)和氢氧化物溶液(0.01–3 M,25和40°C)中测定了甲虫胺水解的动力学。在酸性条件下,使用二阶特定酸催化常数构建哈米特图。的邻使用藤田-西冈方法进行分析的效果。在碱性溶液中,水解显示出氢氧化物浓度的一阶和二阶依赖性。特定的基本催化常数用于构建Hammett图。评估了邻位效应对氢氧化物浓度的一级依赖性。甲酰苯胺通过特定的酸催化在酸性溶液中水解,动力学研究结果与A AC一致2机制。邻位取代由于共振的空间抑制,由于空间体积的阻滞以及空间相互作用而导致反应速率降低。碱性溶液中的主要水解途径与修饰的B AC 2机理一致。在0.10 M氢氧化钠溶液中,间位和对位取代的甲虫胺水解的哈米特图没有显示出取代基效应。然而,邻位取代导致速率常数的降低与取代基的空间体积成正比。
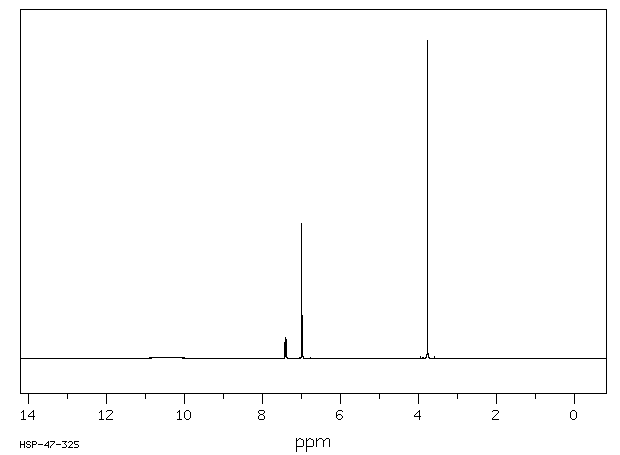
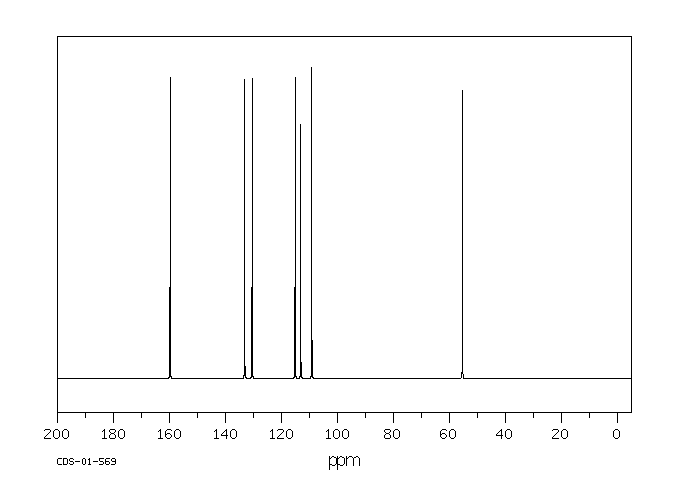
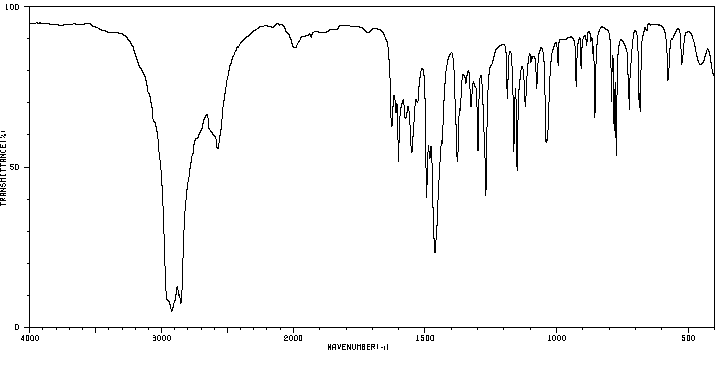
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯