左旋多巴 | 59-92-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:276-278 °C (lit.)
-
比旋光度:-11.7 º (c=5.3, 1N HCl)
-
沸点:334.28°C (rough estimate)
-
密度:1.3075 (rough estimate)
-
溶解度:微溶于水,几乎不溶于乙醇(96%)。它易溶于1M盐酸,微溶于0.1M盐酸。
-
LogP:-1.154 (est)
-
物理描述:Solid
-
颜色/状态:Colorless to white crystals or crystalline powder; needles from water
-
气味:Odorless
-
味道:Tasteless
-
稳定性/保质期:
-
旋光度:Specific optical rotation: -13.1 deg @ 13 °C/D (c= 5.12 in 1 N HCl); max absorption (0.001 N HCl): 220.5 nm (log e= 3.79); 280 nm (log e= 3.42)
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
-
解离常数:pKa = 2.32
-
碰撞截面:148.7 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):-2.7
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:104
-
氢给体数:4
-
氢受体数:5
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S24/25,S26,S36
-
危险类别码:R22,R20/21/22,R36/37/38
-
WGK Germany:3
-
海关编码:2932999099
-
危险品运输编号:OTH
-
RTECS号:AY5600000
-
危险标志:GHS07
-
危险性描述:H302,H315,H319,H335
-
危险性防范说明:P280,P301 + P312 + P330,P304 + P340 + P312,P305 + P351 + P338,P337 + P313
-
储存条件:本品应充入氩气并密封保存于干燥、避光的地方。
SDS
模块 1. 化学品
1.1 产品标识符
: 左旋多巴
产品名称
1.2 鉴别的其他方法
L-3-HydroxytyrOSine
LevoDOPa
3-(3,4-Dihydroxyphenyl)-L-alanine
L-DOPA
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: L-3-HydroxytyrOSine
别名
LevoDOPa
3-(3,4-Dihydroxyphenyl)-L-alanine
L-DOPA
: C9H11NO4
分子式
: 197.19 g/mol
分子量
组分 浓度或浓度范围
LevoDOPa
-
CAS 号 59-92-7
EC-编号 200-445-2
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
恶心, 头痛, 呕吐
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
对光和空气敏感
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 276 - 278 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
空气 发光。
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 1,780 mg/kg
备注: 行为的:兴奋。 行为的:运动失调症 行为的:供给行为
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
恶心, 头痛, 呕吐
附加说明
化学物质毒性作用登记: AY5600000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
左旋多巴是多巴胺的前体物质,在国际上被广泛称为其通用药物名称。不同厂家在药品上市时会有不同的商品名。口服左旋多巴片剂一般会在胃内崩解溶解后,通过十二指肠到达小肠,在小肠上端吸收进入血液,仅有少量最终能透过“血脑屏障”进入大脑,被黑质神经细胞或其他神经细胞摄取。在多巴脱羧酶的作用下,左旋多巴脱去一个羧基,转化为多巴胺,从而补充脑内多巴胺、减轻帕金森病症状。
性状左旋多巴为类白色粉末,无味且无苦。
应用左旋多巴能够治疗帕金森病及帕金森综合征,并可用于治疗肝昏迷,改善中枢神经功能使病人清醒并恢复。此外,它还能促进睡眠、减少脂肪;增加骨密度和逆转骨质疏松;增强肌力与性能力。
副反应常见的副作用包括胃肠道反应:约80%患者在早期服用后会出现恶心及食欲减退等现象,主要是由于多巴胺直接刺激胃肠道或兴奋延脑催吐化学感受区所致。通过餐后服药、缓慢增加剂量或使用多潘立酮(吗丁林)治疗可以减轻症状。
另外,约30%患者可能会出现心血管反应:早期轻度体位性低血压,继续用药可逐渐缓解;还有心律不齐等现象。
化学性质左旋多巴是一种白色或类白色的结晶性粉末,熔点为285.5℃(分解),易溶于稀酸、微溶于水且不溶于乙醇、乙醚和氯仿。无臭、无味,在空气中会变黑。
用途作为目前治疗震颤麻痹的有效药物之一,左旋多巴是体内合成去甲肾上腺素及多巴胺等的前体之一,具有儿茶酚胺特性。它可以透过血脑屏障进入大脑,经多巴脱羧酶作用转化为多巴胺而发挥作用。
生产方法从一些豆科植物中提取出左旋多巴:将藜(Mucuna sempervirens Hemsl)种子粉碎后,在30%乙醇与0.1%乙酸混合液中常温下提取三次,每次24小时。过滤后得到的提取液需减压浓缩,并析出结晶;在0-10℃条件下静置过夜后进行过滤。然后用1N盐酸溶解滤饼,并添加少量维生素C,在pH值为3.5时中和后再析出大量结晶,于0-10℃条件下静置4小时后过滤。滤饼需用水洗两次并用丙酮淋洗脱水处理,在60-70℃下干燥得到左旋多巴成品;豆粉的收率约为2%。
另一种生产方法是通过L-酪氨酸氧化获得:将酪氨酸溶于甲酸磷酸中,升温至40℃保温12小时后,用20倍体积的蒸馏水稀释。然后经强酸性苯乙烯系阳离子交换树脂吸附未反应的酪氨酸,并经过后续处理得到成品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 DL-3-(3,4-二羟苯基)丙氨酸 dopa 63-84-3 C9H11NO4 197.191 盐酸左旋多巴甲酯 L-DOPA methyl ester 7101-51-1 C10H13NO4 211.218 —— (+/-)-3-(3,4-dihydroxyphenyl)-2-methyl-DL-alanine 7101-51-1 C10H13NO4 211.218 L-M-酪氨酸 m-tyrosine 587-33-7 C9H11NO3 181.191 2(S)-氨基-3-(3,4-二羟基苯基)丙酸乙酯 L-DOPA ethyl ester 37178-37-3 C11H15NO4 225.244 (2S)-2-氨基-3-(3,4-二羟基苯基)丙酸异丙酯 L-3,4-dihydroxyphenylalanine isopropyl ester 110301-07-0 C12H17NO4 239.271 L-酪氨酸 L-tyrosine 60-18-4 C9H11NO3 181.191 —— L-3,4-dihydroxyphenylalanine tert-butyl ester 156275-69-3 C13H19NO4 253.298 —— S(-)-carbidopa —— C10H14N2O4 226.232 —— L-3,4-dihydroxyphenylalanine sec-butyl ester —— C13H19NO4 253.298 3,4-二羟基-L-苯丙氨酸苄基酯 L-3,4-dihydroxyphenylalanine benzyl ester 55720-47-3 C16H17NO4 287.315 —— phenoxyethyl ester of L-3,4-dihydroxyphenylalanine 99877-11-9 C17H19NO5 317.342 —— p-methoxyphenylethyl ester of L-3,4-dihydroxyphenylalanine 99877-10-8 C18H21NO5 331.368 —— L-3,4-dihydroxyphenylalanine 2-(tetrahydrofuranyl)methyl ester —— C14H19NO5 281.309 L-苯丙氨酸 L-phenylalanine 63-91-2 C9H11NO2 165.192 多巴胺 3,4-dihydroxyphenylethylamine 51-61-6 C8H11NO2 153.181 N-叔丁氧羰基-3,4-二羟基-L-苯丙氨酸 (2S)-3-(3,4-dihydroxyphenyl)-2-[(tert-butoxy)carbonylamino]propanoic acid 30033-24-0 C14H19NO6 297.308 3-氯-L-酪氨酸 3-chloro-L-tyrosine 7423-93-0 C9H10ClNO3 215.636 —— 3,4-bis(tert-butyldimethylsilyloxy)-L-phenylalanine 161392-58-1 C21H39NO4Si2 425.716 3-溴-L-酪氨酸 3-bromo-L-tyrosine 38739-13-8 C9H10BrNO3 260.087 (S)-(+)-N-乙酰基-3-(4-乙酰氧基-3-甲氧基苯基)丙氨酸 N-acetyl-3-(4-acetoxy-3-methoxyphenyl)alanine 31269-52-0 C14H17NO6 295.292 去甲肾上腺素 norepinephrine 51-41-2 C8H11NO3 169.18 —— N-benzoyl-3-(4-hydroxy-3-methoxyphenyl)-(S)-alanine 30037-41-3 C17H17NO5 315.326 肾上腺素 L-epinephrine 51-43-4 C9H13NO3 183.207 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 DL-3-(3,4-二羟苯基)丙氨酸 dopa 63-84-3 C9H11NO4 197.191 盐酸左旋多巴甲酯 L-DOPA methyl ester 7101-51-1 C10H13NO4 211.218 3-(3-羟基-4-甲氧基苯基)-L-丙氨酸 3-(3-hydroxy-4-methoxyphenyl)-L-alanine 35296-56-1 C10H13NO4 211.218 2(S)-氨基-3-(3,4-二羟基苯基)丙酸乙酯 L-DOPA ethyl ester 37178-37-3 C11H15NO4 225.244 3-甲氧基-L-酪氨酸 3-O-methyldopa 300-48-1 C10H13NO4 211.218 —— L-dopa propyl ester 39638-51-2 C12H17NO4 239.271 3,4-二甲氧基-L-苯丙氨酸 (S)-3,4-dimethoxyphenylalanine 32161-30-1 C11H15NO4 225.244 (2S)-2-氨基-3-(3,4-二羟基苯基)丙酸异丙酯 L-3,4-dihydroxyphenylalanine isopropyl ester 110301-07-0 C12H17NO4 239.271 3,4-二羟基苯基丙酸 dihydrocaffeic acid 1078-61-1 C9H10O4 182.176 L-酪氨酸 L-tyrosine 60-18-4 C9H11NO3 181.191 —— L-3,4-dihydroxyphenylalanine tert-butyl ester 156275-69-3 C13H19NO4 253.298 —— L-dopamide —— C9H12N2O3 196.206 —— 2-amino-3-(3,4-dihydroxyphenyl)propanamide 73148-96-6 C9H12N2O3 196.206 —— S(-)-carbidopa —— C10H14N2O4 226.232 —— L-3,4-dihydroxyphenylalanine sec-butyl ester —— C13H19NO4 253.298 (S)-3,4-二甲氧基苯基丙氨酸甲酯 L-3,4-dimethoxyphenylalanine methyl ester 78083-80-4 C12H17NO4 239.271 —— 2-amino-2-deutero-3-(3,4-dihydroxyphenyl)propionic acid 27261-00-3 C9H11NO4 198.183 (2S)-2-乙酰氨基-3-(3,4-二羟基苯基)丙酸 (S)-3,4-dihydroxyphenylalanine 19641-90-8 C11H13NO5 239.228 —— [18F]-L-3,4-dihydroxy-5-fluorophenylalanine —— C9H10FNO4 214.183 —— (2S)-2-amino-3-(3,4-dimethoxyphenyl)-1-propanol 80582-39-4 C11H17NO3 211.261 —— (2S)-2-amino-3-(2-bromo-4,5-dihydroxyphenyl)propanoic acid 4493-10-1 C9H10BrNO4 276.087 —— (S)-2-Amino-3-benzo[1,3]dioxol-5-yl-propionic acid methyl ester 272784-77-7 C11H13NO4 223.229 —— N-formyl-3,4-dimethoxy-L-phenylalanine 53053-92-2 C12H15NO5 253.255 氟多巴 [18F]-L-3,4-dihydroxy-6-fluorophenylalanine 92812-82-3 C9H10FNO4 214.183 6-氟L-多巴 6-fluoro-L-3,4-dihydroxyphenylalanine 75290-51-6 C9H10FNO4 215.181 4-O-三甲基乙酰基-3-羟基-L-戊胺 L-3-(3-hydroxy-4-pivaloyloxyphenyl)alanine 122551-95-5 C14H19NO5 281.309 3,4-二羟基苯基丙酮酸 3,4-dihydroxyphenylpyruvic acid 4228-66-4 C9H8O5 196.16 —— (S)-methyl 2-amino-3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)propanoate 1213330-83-6 C12H15NO4 237.255 —— S-(-)-3-(3,4-Dihydroxyphenyl)lactic acid 42085-50-7 C9H10O5 198.175 —— L-3,4-dihydroxyphenylalanine 2-(tetrahydrofuranyl)methyl ester —— C14H19NO5 281.309 —— [18F]-L-3,4-dihydroxy-2-fluorophenylalanine —— C9H10FNO4 214.183 —— N-acetyl-3,4-dihydroxyphenylalanine methyl ester 39740-33-5 C12H15NO5 253.255 L-苯丙氨酸 L-phenylalanine 63-91-2 C9H11NO2 165.192 多巴胺 3,4-dihydroxyphenylethylamine 51-61-6 C8H11NO2 153.181 —— L-3,4-dihydroxyphenylalanine 3-tetrahydrofuranyl ester —— C13H17NO5 267.282 —— dihydroclovamide —— C18H19NO7 361.351 —— Nα-trifluoroacetyl-L-DOPA 297144-73-1 C11H10F3NO5 293.199 —— 2-amino-3-(3,4-dihydroxy-phenyl)-propionic acid 2-oxo-2-phenyl-ethyl ester —— C17H17NO5 315.326 —— H-DOPA(acetonide)-OH —— C12H15NO4 237.255 (2S)-2-[[(2S)-2-氨基丙烷酰基]氨基]-3-(3,4-二羟基苯基)丙酸 Ala-L-DOPA 37181-64-9 C12H16N2O5 268.269 N-叔丁氧羰基-3,4-二羟基-L-苯丙氨酸 (2S)-3-(3,4-dihydroxyphenyl)-2-[(tert-butoxy)carbonylamino]propanoic acid 30033-24-0 C14H19NO6 297.308 —— 2-(tert-butoxycarbonylamino)-3-(3,4-dihydroxyphenyl)-propionic acid 59686-55-4 C14H19NO6 297.308 —— 3-hydroxy-N-(DL-3-mercapto-2-methyl-1-oxopropyl)-L-tyrosine 72634-66-3 C13H17NO5S 299.348 —— 3,4-bis(tert-butyldimethylsilyloxy)-L-phenylalanine 161392-58-1 C21H39NO4Si2 425.716 —— methyl 2-chloroacetamido-3-(3,4-dihydroxyphenyl)propanoate —— C12H14ClNO5 287.7 —— 3-hydroxy-N-(3-acetylthiopropanoyl)-L-tyrosine —— C14H17NO6S 327.358 —— L-(3,4-acetonide-dihydroxy)phenylalanine methyl ester 1064196-08-2 C13H17NO4 251.282 —— (S)-2-(((benzyloxy)carbonyl)amino)-3-(3,4-dihydroxyphenyl)propanoic acid 30033-25-1 C17H17NO6 331.325 3-吡啶甲醇,2-溴-6-(三氟甲基)- γ-glutamyl L-3,4-dihydroxyphenylalanine 52370-58-8 C14H18N2O7 326.306 —— (-)-N-[4'-hydroxy-(E)-cinnamoyl]-3-hydroxy-L-tyrosine 77201-64-0 C18H17NO6 343.336 N-BOC-3-羟基-L-酪氨酸甲酯 methyl (S)-2-(tert-butoxycarbonylamino)-3-(3,4-dihydroxyphenyl)propanoate 37169-36-1 C15H21NO6 311.335 —— methyl (2S)-3-(3,4-dihydroxyphenyl)-2-(2,2,2-trifluoroacetamido)propanoate 297144-74-2 C12H12F3NO5 307.226 —— Phe-L-DOPA —— C18H20N2O5 344.367 —— (-)-3,4-Methylenedioxymethamphetamine 81262-70-6 C11H15NO2 193.246 —— 3,4-bis(tert-butyldimethylsilyloxy)-L-phenylalanine methyl ester 161392-59-2 C22H41NO4Si2 439.743 —— L-dopa-L-phenylalanine —— C18H20N2O5 344.367 N-[3',4'-二羟基-(E)-肉桂酰]-3-羟基-L-酪氨酸 (S,E)-3-(3,4-dihydroxyphenyl)-2-(3-(3,4-dihydroxyphenyl)acrylamido)propionic acid 53755-02-5 C18H17NO7 359.335 —— (S)-3-(3,4-dimethoxyphenyl)-2-bromopropanoic acid 151888-22-1 C11H13BrO4 289.126 —— Leu-L-DOPA 37181-65-0 C15H22N2O5 310.35 —— N-(DL-3-acetylthio-2-methyl-1-oxopropyl)-3-hydroxy-L-tyrosine 83591-43-9 C15H19NO6S 341.385 —— N-(tert-butoxycarbonyl)-3,4-(dihydroxy)-L-phenylalanine ethyl ester 1313214-06-0 C16H23NO6 325.362 N-Boc-3,4-二甲氧基-L-苯丙氨酸 (S)‐2‐[(tert‐butoxycarbonyl)amino]‐3‐(3,4‐dimethoxyphenyl)propanoic acid 127095-97-0 C16H23NO6 325.362 —— (3S)-6,7-Dihydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid 34312-81-7 C10H11NO4 209.202 —— L-3-carboxy-7,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline —— C10H11NO4 209.202 —— (S)-methyl 2-(benzyloxycarbonylamino)-3-(3,4-dihydroxyphenyl)propanoate 37169-46-3 C18H19NO6 345.352 去甲肾上腺素 norepinephrine 51-41-2 C8H11NO3 169.18 2-(3,4-二羟基苯基)乙醛 3,4-Dihydroxyphenylacetaldehyde 5707-55-1 C8H8O3 152.15 —— cinnamoyl-DOPA methyl ester 1418029-43-2 C19H19NO5 341.364 —— methyl 3-(3,4-dihydroxyphenyl)-(2S)-2-((2E)-3-(4-hydroxyphenyl)acrylamido)propanoate 1418029-45-4 C19H19NO6 357.363 羟基酪醇 hydroxytyrosol 10597-60-1 C8H10O3 154.166 —— (2S)-3-(3,4-dihydroxyphenyl)-2-(tritylamino)propanoic acid 1261297-76-0 C28H25NO4 439.511 —— (2S)-3-(3,4-dihydroxyphenyl)-2-[[4-[3-[(6Z,9Z,12Z)-octadeca-6,9,12-trienoyl]oxypropoxy]-4-oxobutanoyl]amino]propanoic acid 1243300-36-8 C34H49NO9 615.764 —— (S)-2-tert-Butoxycarbonylamino-3-(3,4-dihydroxy-phenyl)-propionic acid 2-[(S)-2-tert-butoxycarbonylamino-3-(3,4-dihydroxy-phenyl)-propionyloxy]-ethyl ester 913259-56-0 C30H40N2O12 620.654 (S)-4-(3,4-二甲氧基苄基)恶唑烷-2-酮 (S)-4-(3,4-dimethoxybenzyl)oxazolidin-2-one 142763-11-9 C12H15NO4 237.255 肾上腺素 L-epinephrine 51-43-4 C9H13NO3 183.207 —— (2S)-3-(2H-1,3-benzodioxol-5-yl)-2-[(tert-butoxycarbonyl)amino]propanoic acid —— C15H19NO6 309.319 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
反应信息
-
作为反应物:描述:左旋多巴 在 nicotinamide N-methyltransferase 、 phenylalanine decarboxylase 、 dopamine β-hydroxylase 、 氧气 、 维生素 C 、 2-氨基-1-苯乙醇 作用下, 生成 肾上腺素参考文献:名称:플라즈마 중합을 이용한 폴리도파민 합성방법摘要:本发明涉及利用等离子体聚合法制备儿茶酚胺类化合物的方法,具体而言,利用干法等离子体聚合法从苯酚(phenol)或苯胺(aniline)等儿茶酚前体物质制备各种儿茶酚,即具有苯环的邻位(ortho)-羟基(-OH)和对位(para)-羟基的各种烷基胺的单分子化合物的方法。公开号:KR20150094532A
-
作为产物:参考文献:名称:一种左旋多巴的制备方法摘要:本发明公开了一种左旋多巴的制备方法。它在无溶剂条件下,3,4‑二甲氧基苯乙醛、L‑苯甘氨醇和TMSCN在催化剂A的催化作用下,进行三组分“一锅法”不对称硅腈化反应制备得到化合物(III);化合物(III)在酸性条件下水解反应制备得到化合物(IV);化合物(IV)经催化氢化脱除手性辅助基得到左旋多巴。本发明采用廉价且环境友好型催化剂路易斯酸性镁催化硅腈化反应制备左旋多巴,具有高度的立体选择性,不需要低温及无水、无氧等苛刻的操作条件;另外用低毒、安全的三甲基硅氰替代剧毒试剂氰化钠或氰化钾,提高了生产上的安全性,减少了环境污染;具有反应条件温和、操作简便、原子利用率高、环境友好、生产成本低等优点,适于工业化推广应用。公开号:CN107382755A
-
作为试剂:描述:7-羟基-10-氧化物-3H-吩恶嗪-3-酮 在 左旋多巴 、 potassium carbonate 作用下, 以 aq. phosphate buffer 、 N,N-二甲基甲酰胺 为溶剂, 反应 1.0h, 生成参考文献:名称:A new approach for turn-on fluorescence sensing of l-DOPA摘要:Resa-Sulf,基于氧化还原反应设计,被应用于打开荧光感应和定量检测l-DOPA。DOI:10.1039/c7cc07640a
文献信息
-
[EN] TARGETED DELIVERY AND PRODRUG DESIGNS FOR PLATINUM-ACRIDINE ANTI-CANCER COMPOUNDS AND METHODS THEREOF<br/>[FR] ADMINISTRATION CIBLÉE ET CONCEPTIONS DE PROMÉDICAMENTS POUR COMPOSÉS ANTICANCÉREUX À BASE DE PLATINE ET D'ACRIDINE ET MÉTHODES ASSOCIÉES申请人:WAKE FOREST SCHOOL OF MEDICINE公开号:WO2013033430A1公开(公告)日:2013-03-07Acridine containing cispiaiin compounds have been disclosed that show greater efficacy against cancer than other cisplatin compounds. Methods of delivery of those more effective eisp!aiin compounds to the nucleus in cancer ceils is disclosed using one or more amino acids, one or more sugars, one or more polymeric ethers, C i^aikylene-phenyl-NH-C(0)-R.15, folic acid, av03 iniegriii RGD binding peptide, tamoxifen, endoxifen, epidermal growth factor receptor, antibody conjugates, kinase inhibitors, diazoles, triazol.es, oxazoies, erlotinib, and/or mixtures thereof; wherein R]§ is a peptide.
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] COMPOUNDS FOR THE TREATMENT OF AMYLOID-ASSOCIATED DISEASES<br/>[FR] COMPOSÉS POUR LE TRAITEMENT DE MALADIES ASSOCIÉES À LA SUBSTANCE AMYLOÏDE申请人:REMYND NV公开号:WO2016083490A1公开(公告)日:2016-06-02This invention provides novel compounds of formulae (I) or (II) or a stereoisomer, enantiomer, racemic, or tautomer thereof, (I) (II) wherein the substituents are as defined in the specification. The present invention also relates to the novel compounds for use as a medicine, more in particular for the prevention or treatment of amyloid-related diseases, more specifically certain neurological disorders, such as disorders collectively known as tauopathies, disorders characterized by cytotoxic α-synuclein amyloidogenesis. The present invention also relates to the use of said novel compounds for the manufacture of medicaments useful for treating such amyloid-related diseases. The present invention further relates to pharmaceutical compositions including said novel compounds and to methods for the preparation of said novel compounds.这项发明提供了式(I)或(II)或其立体异构体、对映异构体、消旋体或互变异构体的新化合物,其中取代基如规范中所定义。本发明还涉及用作药物的这些新化合物,更具体地用于预防或治疗与淀粉样蛋白相关的疾病,更具体地说是某些神经系统疾病,如被统称为tau病变的疾病,以及由细胞毒性α-突触核蛋白淀粉生成所特征化的疾病。本发明还涉及利用这些新化合物制备对治疗此类淀粉样蛋白相关疾病有用的药物。本发明还涉及包括这些新化合物的药物组合物以及这些新化合物的制备方法。
-
Chromenone derivatives useful for the treatment of neurodegenerative diseases申请人:AxoGlia Therapeutics S.A.公开号:EP2112145A1公开(公告)日:2009-10-28Compounds of general formula (I) and (II) in which R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, R13, R14 and R15 have the meanings given in the specification, are useful in the treatment of neurodegenerative disease.通式(I)和(II)的化合物 其中R1、R2、R3、R4、R5、R6、R7、R8、R9、R10、R11、R12、R13、R14和R15具有规范中给定的含义,在神经退行性疾病的治疗中是有用的。
-
[EN] SUBSTITUTED N-HETEROCYCLIC CARBOXAMIDES AS ACID CERAMIDASE INHIBITORS AND THEIR USE AS MEDICAMENTS<br/>[FR] CARBOXAMIDES N-HÉTÉROCYCLIQUES SUBSTITUÉS UTILISÉS EN TANT QU'INHIBITEURS DE LA CÉRAMIDASE ACIDE ET LEUR UTILISATION EN TANT QUE MÉDICAMENTS申请人:BIAL BIOTECH INVEST INC公开号:WO2021055627A1公开(公告)日:2021-03-25The invention provides substituted N-heterocyclic carboxamides and related compounds, compositions containing such compounds, medical kits, and methods for using such compounds and compositions to treat a medical disorder, e.g., cancer, lysosomal storage disorder, neurodegenerative disorder, inflammatory disorder, in a patient.这项发明提供了替代的N-杂环羧酰胺和相关化合物,含有这些化合物的组合物,医疗工具包,以及使用这些化合物和组合物治疗患者的医疗疾病(例如癌症、溶酶体贮积症、神经退行性疾病、炎症性疾病)的方法。
表征谱图
-
氢谱1HNMR
-
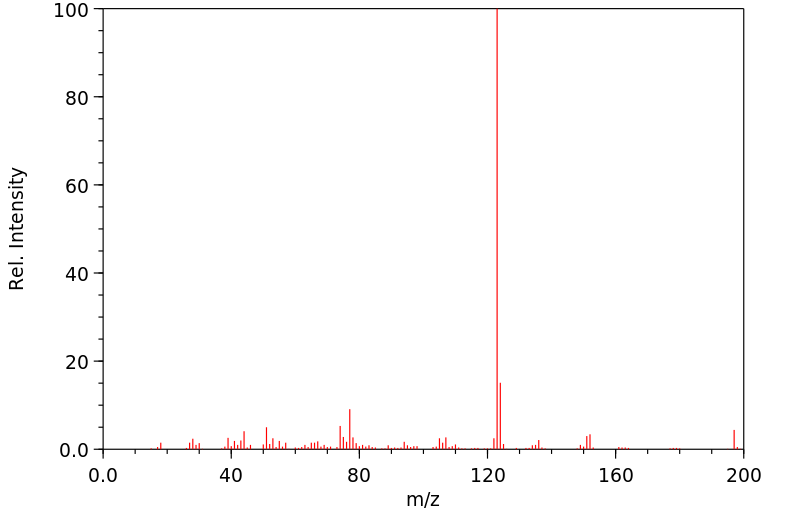
质谱MS
-
碳谱13CNMR
-
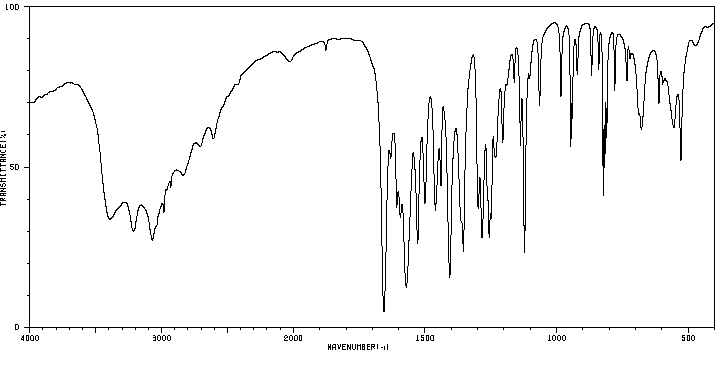
红外IR
-
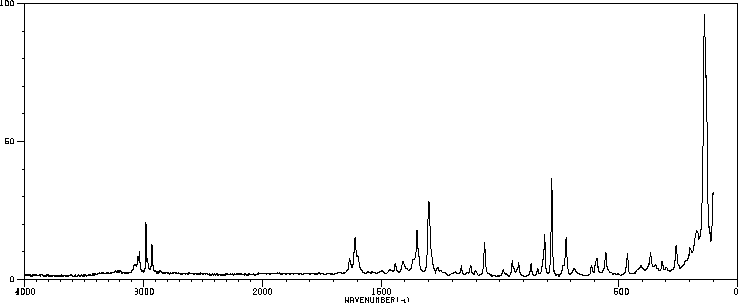
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息