1-甲基靛红 | 2058-74-4
中文名称
1-甲基靛红
中文别名
1-甲基吲哚-2,3-二酮;N-甲基靛红;N-甲基靛紅;1-甲基-2,3-吲哚二酮
英文名称
1-methyl-1H-indole-2,3-dione
英文别名
N-methylisatin;1-methylindoline-2,3-dione;1-methylisatin;1-methylindole-2,3-dione
CAS
2058-74-4
化学式
C9H7NO2
mdl
MFCD00005812
分子量
161.16
InChiKey
VCYBVWFTGAZHGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:130-133 °C (lit.)
-
沸点:287.44°C (rough estimate)
-
密度:1.314
-
最大波长(λmax):427nm(CH2Cl2)(lit.)
-
保留指数:1477
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解,也未有已知危险反应。应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.111
-
拓扑面积:37.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:T
-
危险类别码:R25
-
危险品运输编号:UN 2811 6.1/PG 3
-
WGK Germany:3
-
RTECS号:NL7939000
-
海关编码:2933990090
-
安全说明:S26,S39,S45
-
危险标志:GHS05,GHS06
-
危险性描述:H301,H315,H318,H335
-
危险性防范说明:P261,P280,P301 + P310,P305 + P351 + P338
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境具备良好的通风或排气设施。
SDS
| Name: | 1-Methylisatin 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2058-74-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2058-74-4 | 1-Methylisatin | 98% | 218-164-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2058-74-4: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: orange to brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 129-133 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H7NO2
Molecular Weight: 161.16
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2058-74-4: NL7939000 LD50/LC50:
Not available.
Carcinogenicity:
1-Methylisatin - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 2058-74-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2058-74-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2058-74-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-乙基-1H-吲哚-2,3-二酮 N-ethylisatin 4290-94-2 C10H9NO2 175.187 6-溴-1-甲基吲哚-2,3-二酮 6-bromo-N-methylisatin 667463-64-1 C9H6BrNO2 240.056 靛红 indole-2,3-dione 91-56-5 C8H5NO2 147.133 1-苄基-1H-吲哚-2,3-二酮 1-benzylisatin 1217-89-6 C15H11NO2 237.258 N-甲基吲哚酮 N-methyl-2-indolinone 61-70-1 C9H9NO 147.177 —— N-benzyl-5-iodoisatin 625456-97-5 C15H10INO2 363.154 2-亚氨基-1-甲基-1,2-二氢-吲哚-3-酮 2-imino-1-methyl-1,2-dihydro-indol-3-one 71653-53-7 C9H8N2O 160.175 —— 3-hydroxy-1-methyl-indolin-2-one 3335-86-2 C9H9NO2 163.176 —— 3-bromo-1-methylindolin-2-one 3265-27-8 C9H8BrNO 226.073 —— 1-Methyl-3-methylthio-2-oxindol 40800-68-8 C10H11NOS 193.269 —— 3-(α-15N)diazo-1-methylindolin-2-one —— C9H7N3O 174.167 —— 3,3-dichloro-1-methylindolin-2-one 114380-33-5 C9H7Cl2NO 216.067 —— (Z)-2-benzylidene-1-methylindolin-3-one 38072-57-0 C16H13NO 235.285 2-吲哚酮 2-oxoindole 59-48-3 C8H7NO 133.15 3-苄基-1,3-二氢-1-甲基-2H-吲哚-2-酮 3-benzyl-1-methyl-1,3-dihydroindol-2-one 3335-85-1 C16H15NO 237.301 —— 1-methyl-3-phenylsulfanylindol-2(3H)-one 2406-10-2 C15H13NOS 255.34 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-乙基-1H-吲哚-2,3-二酮 N-ethylisatin 4290-94-2 C10H9NO2 175.187 5-氯-1-甲基-1H-吲哚-2,3-二酮 5-chloro-1-methylindoline-2, 3-dione 60434-13-1 C9H6ClNO2 195.605 1-(羟基甲基)-吲哚-3-二酮 N-Hydroxymethylisatin 50899-59-7 C9H7NO3 177.159 —— 5-iodo-N-methylisatin 76034-84-9 C9H6INO2 287.057 5-溴-1-甲基-1H-吲哚-2,3-二酮 5-bromo-1-methyl-1H-indole-2,3-dione 2058-72-2 C9H6BrNO2 240.056 1-甲基-5-硝基靛红 1-methyl-5-nitro-1H-indole-2,3-dione 3484-32-0 C9H6N2O4 206.158 1-苄基-1H-吲哚-2,3-二酮 1-benzylisatin 1217-89-6 C15H11NO2 237.258 N-甲基吲哚酮 N-methyl-2-indolinone 61-70-1 C9H9NO 147.177 5-羟基-1-甲基-2-吲哚啉酮 1-methyl-5-hydroxy-2-indolinone 6062-24-4 C9H9NO2 163.176 1,3-二甲基-1,3-二氢-2H-吲哚-2-酮 1,3-dimethylindolin-2-one 24438-17-3 C10H11NO 161.203 1-甲基-3-亚甲基-1,3-二氢-2H-吲哚-2-酮 1-methyl-3-methylene-1,3-dihydro-2H-indol-2-one 59181-28-1 C10H9NO 159.188 3-氨基-1-甲基-1,3-二氢-2H-吲哚-2-酮 3-amino-1-methylindolin-2-one 121974-35-4 C9H10N2O 162.191 —— 1-methyl-4-hydroxyoxindole 20870-81-9 C9H9NO2 163.176 —— 3-hydroxy-1-methyl-indolin-2-one 3335-86-2 C9H9NO2 163.176 —— 3-bromo-1-methylindolin-2-one 3265-27-8 C9H8BrNO 226.073 —— (R)-3-hydroxy-1-methyl-1,3-dihydro-indol-2-one 124508-46-9 C9H9NO2 163.176 —— (S)-3-hydroxy-1-methyl-1,3-dihydro-indol-2-one 1126736-27-3 C9H9NO2 163.176 1-苯基靛红 1-phenyl-indole-2,3-dione 723-89-7 C14H9NO2 223.231 —— 3,3-difluoro-1-methylindolin-2-one 73789-91-0 C9H7F2NO 183.157 1,3-二氢-1-甲基-3-苯基-2H-吲哚-2-酮 1-methyl-3-phenylindolin-2-one 3335-97-5 C15H13NO 223.274 —— 3-methoxy-1-methylindolin-2-one 57661-12-8 C10H11NO2 177.203 —— 1-methyl-1H-indole-2,3-dione 3-hydrazone 3265-23-4 C9H9N3O 175.19 —— (E)-3-hydrazono-1-methylindolin-2-one —— C9H9N3O 175.19 —— 1-methylisatin 3-hydrazone 3265-23-4 C9H9N3O 175.19 —— 1-methyl-3-(p-tolyl)indolin-2-one —— C16H15NO 237.301 3-乙基-1-甲基-1,3-二氢-2H-吲哚-2-酮 3-ethyl-1-methylindolin-2-one 2525-35-1 C11H13NO 175.23 —— 3-(hydroxyimino)-1-methylindolin-2-one —— C9H8N2O2 176.175 —— N-Methyl-isatin-3-oxim —— C9H8N2O2 176.175 —— (3E)-3-ethylidene-1-methyl-1,3-dihydro-2H-indol-2-one 58807-96-8 C11H11NO 173.214 —— (Z)-3-ethylidene-1-methylindolin-2-one 58807-97-9 C11H11NO 173.214 —— 3,3-dichloro-1-methylindolin-2-one 114380-33-5 C9H7Cl2NO 216.067 —— 3-(4-chlorophenyl)-1-methylindolin-2-one 3335-94-2 C15H12ClNO 257.719 —— 3-allyl-1-methylindolin-2-one 32380-68-0 C12H13NO 187.241 —— 3-(4-methoxyphenyl)-1-methylindolin-2-one 51206-81-6 C16H15NO2 253.301 —— 1-Methyl-3-[4-(trifluoromethyl)phenyl]indoline-2-one 1453221-27-6 C16H12F3NO 291.273 —— 3-(4-fluorophenyl)-1-methylindolin-2-one 845512-35-8 C15H12FNO 241.265 1-甲基-3-(萘-2-基)二氢吲哚-2-酮 1-methyl-3-(naphthalen-2-yl)indolin-2-one 1001163-24-1 C19H15NO 273.334 —— 3-isothiocyanato-1-methylindolin-2-one 1287715-28-9 C10H8N2OS 204.252 —— (E)-2-(1-methyl-2-oxoindolin-3-ylidene)acetonitrile 876140-51-1 C11H8N2O 184.197 —— (1-methyl-2-oxo-indolin-3-yliden)-acetonitrile 118659-23-7 C11H8N2O 184.197 —— N-methylisatin-β-amidinohydrazone 52599-37-8 C10H11N5O 217.23 —— (1-methyl-2-oxo-2,3-dihydro-1H-indol-3-yl)acetonitrile 99984-61-9 C11H10N2O 186.213 2-吲哚酮 2-oxoindole 59-48-3 C8H7NO 133.15 —— (Z)-3-(Methoxyimino)-1-methylindolin-2-one —— C10H10N2O2 190.202 3-苄基-1,3-二氢-1-甲基-2H-吲哚-2-酮 3-benzyl-1-methyl-1,3-dihydroindol-2-one 3335-85-1 C16H15NO 237.301 —— 1-methyl-3-(2-oxo-2-phenylethyl)indolin-2-one 844-49-5 C17H15NO2 265.312 —— 1-methyl-isatin-(3)-semicarbazone 112367-66-5 C10H10N4O2 218.215 —— N-Methylisatin-β-semicarbazon 25673-65-8 C10H10N4O2 218.215 —— (R)-3-(hydroxymethyl)-1,3-dimethylindolin-2-one 685089-44-5 C11H13NO2 191.23 1-甲基-2-氧代-N-苯基-3H-吲哚-3-甲酰胺 1-methyl-2-oxo-N-phenylindoline-3-carboxamide 32866-51-6 C16H14N2O2 266.299 —— 1-methyl-3-(N,N-dimethylamino)methyleneindol-2-one 848649-88-7 C12H14N2O 202.256 —— Methisazon 26153-15-1 C10H10N4OS 234.282 —— 1-Methylisatin 3-thiosemicarbazone 26134-46-3 C10H10N4OS 234.282 美替沙腙 metisazone 1910-68-5 C10H10N4OS 234.282 —— 1-methyl-2-oxo-N-(p-tolyl)indoline-3-carboxamide 32866-55-0 C17H16N2O2 280.326 —— N-methyl-1,3-dihydro-3,3-diphenyl-2H-indol-2-one 22136-54-5 C21H17NO 299.372 —— (E)-3-benzylidene-1-methylindolin-2-one 34219-51-7 C16H13NO 235.285 —— 3-benzylidene-1-methyl-1,3-dihydroindol-2-one 34219-52-8 C16H13NO 235.285 —— 3-benzylidene-1-methylindolin-2-one 59181-30-5 C16H13NO 235.285 —— 1-methyl-3-(2-methylallyl)indolin-2-one 1170697-33-2 C13H15NO 201.268 —— 3,3-bis(hydroxymethyl)-1-methyloxindole 374898-38-1 C11H13NO3 207.229 —— (Z)-3-hexylidene-1-methylindolin-2-one 1194813-24-5 C15H19NO 229.322 —— N-Methylisatin 3-phenylhydrazone 15096-16-9 C15H13N3O 251.288 —— 1-methyl-3-(2-phenylhydrazineylidene)indolin-2-one 15096-16-9 C15H13N3O 251.288 —— (E)-3-(isopropoxyimino)-1-methylindolin-2-one 1167423-21-3 C12H14N2O2 218.255 —— 1-methyl-3-(o-tolyl)indolin-2-one 349081-47-6 C16H15NO 237.301 —— N-(1-methyl-2-oxo-2,3-dihydro-1H-indol-3-yl)-acetamide 855420-26-7 C11H12N2O2 204.228 —— 1-methyl-3-(morpholinoimino)indolin-2-one 95060-49-4 C13H15N3O2 245.281 —— 1-methyl-2-oxo-N-(o-tolyl)indoline-3-carboxamide 32866-56-1 C17H16N2O2 280.326 —— 1'-methylspiro[cyclopropane-1,3'-indolin]-2'-one 14276-11-0 C11H11NO 173.214 —— (Z)-N-methyl-2-(1-methyl-2-oxoindolin-3-ylidene)hydrazinecarbothioamide 53013-78-8 C11H12N4OS 248.308 (1-甲基-2-氧代-2,3-二氢-1H-吲哚-3-基)-乙酸 1-Methyl-2-indolinon-3-essigsaeure 21591-75-3 C11H11NO3 205.213 —— N-methyl-2-(1-methyl-2-oxoindolin-3-yl)acetamide 31829-55-7 C12H14N2O2 218.255 —— Z-3-cyanoacetylhydrazono-1-methyl-2-indolinone 1199911-04-0 C12H10N4O2 242.237 —— (Z)-N’-(1-methyl-2-oxoindolin-3-ylidene)benzohydrazide —— C16H13N3O2 279.298 —— N-[(E)-(1-methyl-2-oxoindol-3-ylidene)amino]benzamide —— C16H13N3O2 279.298 —— 1-methyl-3-(1H-pyrrol-1-yl)indolin-2-one 16176-42-4 C13H12N2O 212.251 —— 2-(4-methylanilino)-N-[(Z)-(1-methyl-2-oxoindol-3-ylidene)amino]acetamide —— C18H18N4O2 322.4 —— (3,3-dimethoxy-N-methyl)indoline-2-one 14271-46-6 C11H13NO3 207.229 —— (Z)-N-methylisatin-3-4-phenyl(semicarbazone) 1421700-44-8 C16H14N4O2 294.313 —— 1-methyl-3-(2-oxo-2-phenylethylidene)indolin-2-one 27230-23-5 C17H13NO2 263.296 —— (E)-1-methyl-3-(2-oxo-2-phenylethylidene)indolin-2-one 56680-30-9 C17H13NO2 263.296 —— (Z)-1-methyl-3-(2-oxo-2-phenylethylidene)dihydroindol-2-one 27230-23-5 C17H13NO2 263.296 —— (E)-1-methyl-3-(2-oxopropylidene)indolin-2-one 70351-51-8 C12H11NO2 201.225 —— supercinnamaldehyde 70351-51-8 C12H11NO2 201.225 (3Z)-1-甲基-3-(2-氧代亚丙基)-1,3-二氢-2H-吲哚-2-酮 1-Methyl-3-[2-oxo-prop-(Z)-ylidene]-1,3-dihydro-indol-2-one 70351-51-8 C12H11NO2 201.225 —— N-(4-methoxyphenyl)-1-methyl-2-oxoindoline-3-carboxamide 32866-53-8 C17H16N2O3 296.326 —— 3,3-bis(4-hydroxyphenyl)-1-methylindolin-2-one 47414-00-6 C21H17NO3 331.371 —— 3-(4-hydroxyphenyl)-1-methyl-3-phenylindolin-2-one 1425058-56-5 C21H17NO2 315.371 —— 1-methylisatin-3-p-chlorophenylhydrazone 113258-69-8 C15H12ClN3O 285.733 —— (Z)-4-methyl-N’-(1-methyl-2-oxoindolin-3-ylidene)benzohydrazide —— C17H15N3O2 293.325 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:低价钛对靛红与酮和醛的还原偶联摘要:Zn-TiCl 4在THF中将靛红与酮和醛还原偶联,可通过控制反应条件选择性地产生两电子还原产物和3-电子还原产物3-羟基-3-(1-羟基烷基)-吲哚和3-烷基亚乙基吲哚。尽管在某些情况下3-(1-羟烷基)恶吲哚也作为四电子还原产物产生,但这些产物容易脱水成3-亚烷基氧杂吲哚。衍生自醛的3-亚烷基氧杂吲哚形成为几何异构体的混合物。通过在催化剂中回流,将两种几何异构体异构化成平衡混合物。PPTS /苯。DOI:10.1016/j.tet.2014.10.071
-
作为产物:描述:参考文献:名称:Stolle et al., Journal fur praktische Chemie (Leipzig 1954), 1930, vol. <2>128, p. 1,23摘要:DOI:
-
作为试剂:参考文献:名称:“水上”合成硫代咪唑啉酮-靛红/茚三酮缀合物,然后通过 ZnMnO3@Ni(OH)2 纳米催化剂进行温度诱导脱水摘要:一种新型高效的“水上”介导的一锅法ZnMnO 3 @Ni(OH) 2催化直接合成3-羟基-3-(3-甲基-5-氧代-2-硫代咪唑烷-4-基)羟吲哚已经探索了通过噻唑烷二酮-靛红缀合物。同时,还证明了3-羟基-3-(3-甲基-5-氧代-2-硫代咪唑啉-4-基)羟吲哚的温度调节脱水加合物。在水介质中使用可回收的ZnMnO 3 @Ni(OH) 2纳米催化剂使该方案具有可持续性和绿色性。使用粉末 XRD、HRTEM、EDX 和 BET 分析对 ZnMnO 3 @Ni(OH) 2纳米粉末的合成进行了细致的表征。此外,在温和且操作简单的反应条件下选择易于获得且易于制备的起始材料是该策略的主要优点。该催化剂具有高水相容性,并具有至少八个循环的回收和再利用能力。通过标准的浸出实验,证实该反应在这种可回收催化剂的作用下进行非均相。DOI:10.1039/d3gc03730d
文献信息
-
Synthesis and biological evaluation of new [1,2,4]triazino[5,6-b]indol-3-ylthio-1,3,5-triazines and [1,2,4]triazino[5,6-b]indol-3-ylthio-pyrimidines against Leishmania donovani作者:Leena Gupta、Naresh Sunduru、Aditya Verma、Saumya Srivastava、Suman Gupta、Neena Goyal、Prem M.S. ChauhanDOI:10.1016/j.ejmech.2010.02.015日期:2010.6series of [1,2,4]triazino[5,6-b]indol-3-ylthio-1,3,5-triazines and [1,2,4]triazino[5,6-b]indol-3-ylthio-pyrimidines were synthesized and screened for their in vitro antileishmanial activity against Leishmania donovani. Among all, 8 compounds have shown more than 90% inhibition against promastigotes and IC50 in the range of 4.01–57.78 μM against amastigotes. Compound 5, a triazino[5,6-b]indol-3-ylthio-1一系列的[1,2,4] triazino [5,6 - b ] indol-3-ylthio-1,3,5-triazines和[1,2,4] triazino [5,6 - b ] indol-3合成了-ylthiothiopyrimidines,并筛选了它们对利什曼原虫的体外抗疟原虫活性。在所有化合物中,有8种化合物对前鞭毛体的抑制作用超过90%,对Amastigotes的IC 50抑制作用在4.01至57.78μM之间。化合物5,一个嗪[5,6- b ]吲哚-3-基硫基-1,3,5-三嗪衍生物被发现是最活跃的,并用20-&毒性最低的10倍的选择性(SI = 56.61)与标准药物喷他idine和stibogluconate(SSG)相比。
-
Synthesis, Anticancer and Antibacterial Activity of Some Novel Mononuclear Ru(II) Complexes作者:Upal Kanti Mazumder、Malaya Gupta、Subhas Somalingappa Karki、Shiladitya Bhattacharya、Suresh Rathinasamy、Sivakumar ThangavelDOI:10.1248/cpb.52.178日期:——In search of potential anticancer drug candidates in ruthenium complexes, a series of mononuclear ruthenium complexes of the type [Ru(phen)2(nmit)]Cl2 (Ru1), [Ru(bpy)2(nmit)]Cl2 (Ru2), [Ru(phen)2(icpl)]Cl2 (Ru3), Ru(bpy)2(icpl)]Cl2 (Ru4) (phen=1,10-phenanthroline; bpy=2,2′-bipyridine; nmit=N-methyl-isatin-3-thiosemicarbazone, icpl=isatin-3-(4-Cl-phenyl)thiosemicarbazone) and [Ru(phen)2(aze)]Cl2 (Ru5), [Ru(bpy)2(aze)]Cl2 (Ru6) (aze=acetazolamide) and [Ru(phen)2(R-tsc)](ClO4)2 (R=methyl (Ru7), ethyl (Ru8), cyclohexyl (Ru9), 4-Cl-phenyl (10), 4-Br-phenyl (Ru11), and 4-EtO-phenyl (Ru12), tsc=thiosemicarbazone) were prepared and characterized by elemental analysis, FTIR, 1H-NMR and FAB-MS. Effect of these complexes on the growth of a transplantable murine tumor cell line (Ehrlich Ascites Carcinoma) and their antibacterial activity were studied. In cancer study the effect of hematological profile of the tumor hosts have also been studied. In the cancer study, the complexes Ru1—Ru4, Ru10 and Ru11 have remarkably decreased the tumor volume and viable ascitic cell count as indicated by trypan blue dye exclusion test (p<0.05). Treatment with the ruthenium complexes prolonged the lifespan of Ehrlich Ascites Carcinoma (EAC) bearing mice. Tumor inhibition by the ruthenium chelates was followed by improvements in hemoglobin, RBC and WBC values. All the complexes showed antibacterial activity, except Ru5 and Ru6. Thus, the results suggest that these ruthenium complexes have significant antitumor property and antibacterial activity. The results also reflect that the drug does not adversely affect the hematological profiles as compared to that of cisplatin on the host.为了寻找潜在的抗癌药物候选物,合成并表征了一系列单核钌配合物,包括[Ru(phen)2(nmit)]Cl2 (Ru1)、[Ru(bpy)2(nmit)]Cl2 (Ru2)、[Ru(phen)2(icpl)]Cl2 (Ru3)、[Ru(bpy)2(icpl)]Cl2 (Ru4)(其中phen=1,10-菲咯啉,bpy=2,2'-联吡啶,nmit=N-甲基-异氮茚-3-缩氨基硫脲,icpl=异氮茚-3-(4-氯苯基)缩氨基硫脲),以及[Ru(phen)2(aze)]Cl2 (Ru5)、[Ru(bpy)2(aze)]Cl2 (Ru6)(aze=醋唑磺胺)和[Ru(phen)2(R-tsc)](ClO4)2(R=甲基(Ru7)、乙基(Ru8)、环己基(Ru9)、4-氯苯基(Ru10)、4-溴苯基(Ru11)和4-乙氧基苯基(Ru12),tsc=缩氨基硫脲)。研究了这些配合物对可移植的小鼠肿瘤细胞系(埃利希腹水癌)生长的影响及其抗菌活性。在癌症研究中,还研究了肿瘤宿主血液学特征的影响。在癌症研究中,配合物Ru1—Ru4、Ru10和Ru11显著减少了肿瘤体积和活腹水细胞数量,通过台阶蓝染色排斥试验显示(p<0.05)。使用这些钌配合物治疗延长了携带埃利希腹水癌(EAC)小鼠的寿命。钌配合物抑制肿瘤后,血红蛋白、红细胞和白细胞值有所改善。所有配合物都显示出抗菌活性,除了Ru5和Ru6。因此,结果表明这些钌配合物具有显著的抗肿瘤特性和抗菌活性。结果还反映出,与顺铂相比,该药物对宿主的血液学特征没有负面影响。
-
Access to Spirocyclized Oxindoles and Indolenines via Palladium-Catalyzed Cascade Reactions of Propargyl Carbonates with 2-Oxotryptamines and Tryptamines作者:Antoinette E. Nibbs、Thomas D. Montgomery、Ye Zhu、Viresh H. RawalDOI:10.1021/acs.joc.5b00277日期:2015.5.15through a process wherein the first nucleophilic unit on the oxindole or indole reacts with an allenyl-palladium species, formed from oxidative addition of Pd(0) to propargyl carbonates, to generate a π-allyl palladium intermediate that then reacts further with the second nucleophilic component of the oxindole or indole. The cascade process forges two bonds en route to spirocyclized oxindole and indolenine
-
Site-Selective C–H Functionalization of (Hetero)Arenes via Transient, Non-symmetric Iodanes作者:Stacy C. Fosu、Chido M. Hambira、Andrew D. Chen、James R. Fuchs、David A. NagibDOI:10.1016/j.chempr.2018.11.007日期:2019.2A strategy for C–H functionalization of arenes and heteroarenes has been developed to allow site-selective incorporation of various anions, including Cl, Br, OMs, OTs, and OTf. This approach is enabled by in situ generation of reactive, non-symmetric iodanes by combining anions and bench-stable PhI(OAc)2. The utility of this mechanism is demonstrated via para-selective chlorination of medicinally relevant
-
Autoxidation/Aldol Tandem Reaction of 2-Oxindoles with Ketones: A Green Approach for the Synthesis of 3-Hydroxy-2-Oxindoles作者:Qing-Bao Zhang、Wen-Liang Jia、Yong-Liang Ban、Yong Zheng、Qiang Liu、Li-Zhu WuDOI:10.1002/chem.201504282日期:2016.2.18In the presence of tetrabutylammonium fluoride and molecular sieves (MS) 4 Å in DMF, an efficient autoxidation reaction of 2‐oxindoles with ketones under air at room temperature has been developed. This approach may provide a green, practical, and metal‐free protocol for a wide range of biologically important 3‐hydroxy‐3‐(2‐oxo‐alkyl)‐2‐oxindoles.
表征谱图
-
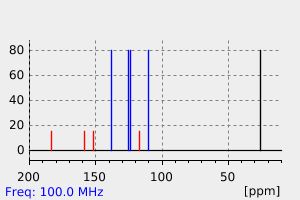
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
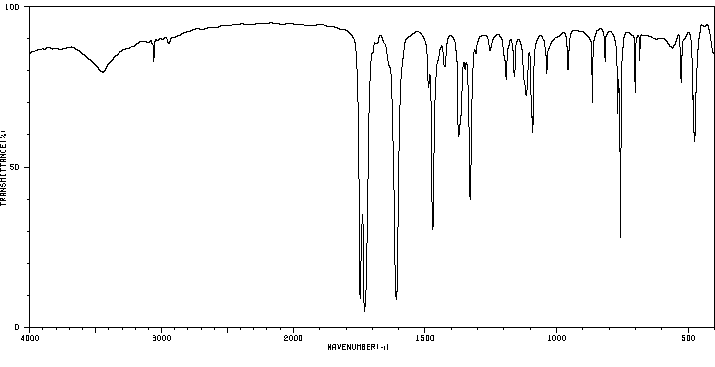
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3