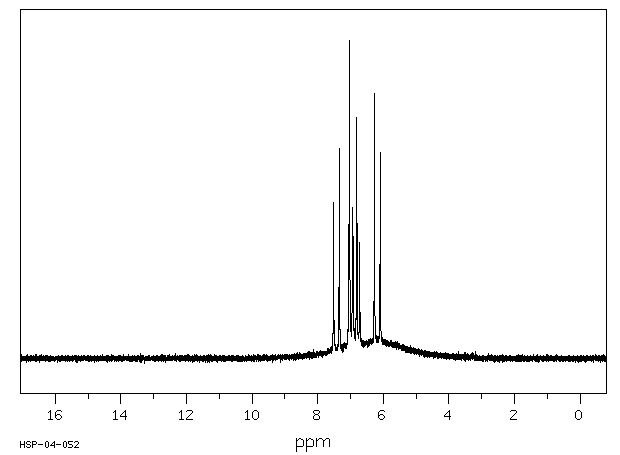
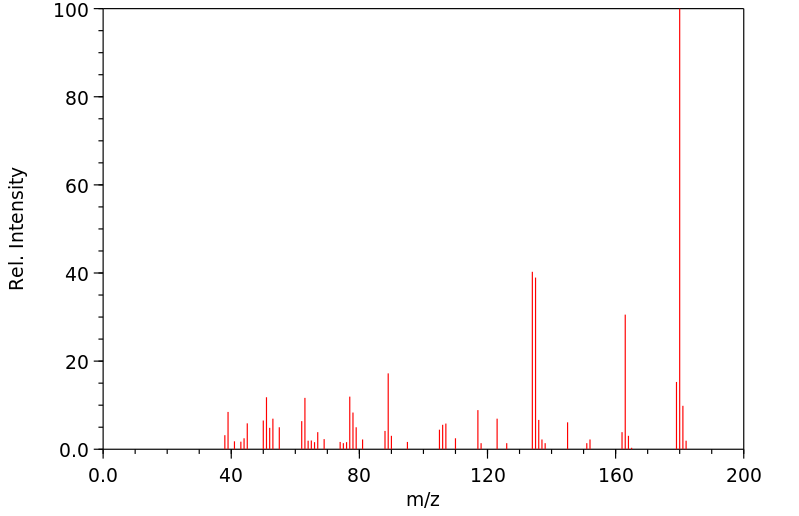
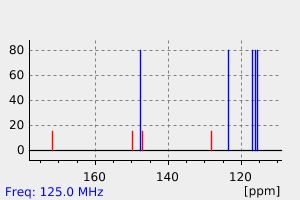
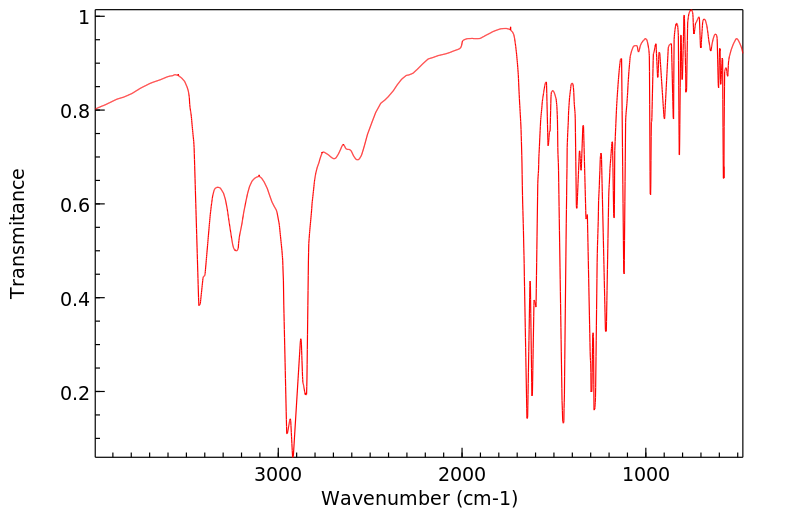
代谢
咖啡酸(caffeic acid)参与的代谢酶尚未被鉴定。在以下实验中,咖啡酸(CA)、绿原酸(CGA)和二氢咖啡酸(DHCA)与肝细胞一起孵育,并显示通过细胞色素P450、儿茶酚-O-甲基转移酶(COMT)和β-氧化酶发生代谢。阿魏酸(FA)或二氢阿魏酸(DHFA),作为COMT催化CA或DHCA-O-甲基化的结果而形成,也可以通过CYP1A1/2进行O-去甲基化,而不是CYP2E1。DHCA或DHFA也经历了侧链脱氢,分别形成CA和FA,这个过程可以被β-氧化酶酰基CoA脱氢酶的抑制剂硫代乙醇酸预防。NADPH/微粒体(CYP2E1)催化的谷胱甘肽结合物形成的速率按降序排列为DHCA>CA>CGA,这与COMT催化的O-甲基化速率的顺序相反。由NADPH/P450形成的CA和DHCA-o-醌可能抑制COMT,但可以容易地形成谷胱甘肽结合物。CA、DHCA和DHFA可以通过隔离的大鼠肝细胞相互代谢以及转化为FA,而FA仅代谢为CA,而不是DHCA或DHFA。CA、DHCA、FA、DHFA和CGA显示出剂量依赖性的肝细胞毒性,测定的LD50(2小时)按有效性降序排列为DHCA>CA>DHFA>CGA>FA。总之,已有证据表明O-甲基化、GSH结合、加氢和脱氢参与CA和DHCA的肝脏代谢。CA和DHCA的O-甲基化途径是一种解毒途径,而P450催化的o-醌形成是毒性途径。
Enzymes involved in its /caffeic acid/ metabolism have not been identified. In the following, caffeic (CA), chlorogenic (CGA), and dihydrocaffeic (DHCA) acids were incubated with hepatocytes and shown to undergo metabolism by cytochrome P450, catechol-O-methyltransferase (COMT), and beta-oxidation enzymes. Ferulic (FA) or dihydroferulic (DHFA) acids, formed as the result of CA- or DHCA-O-methylation by COMT, were also O-demethylated by CYP1A1/2 but not CYP2E1. DHCA or DHFA also underwent side chain dehydrogenation to form CA and FA, respectively, which was prevented by thioglycolic acid, an inhibitor of the beta-oxidation enzyme acyl CoA dehydrogenase. The rates of glutathione conjugate formation catalyzed by NADPH/microsomes (CYP2E1) in decreasing order DHCA>CA>CGA trend which was in reverse order to the rates of their O-methylation by COMT. The CA- and DHCA-o-quinones formed by NADPH/P450 likely inhibited COMT but can readily form glutathione conjugates. CA, DHCA and DHFA were inter-metabolized to each other and to FA by isolated rat hepatocytes whereas FA was metabolized only to CA but not to DHCA or DHFA. CA, DHCA, FA, DHFA and CGA showed a dose-dependent hepatocyte toxicity and the LD(50) (2 h), determined were in decreasing order of effectiveness DHCA>CA>DHFA>CGA>FA. In summary, evidence has been provided that O-methylation, GSH conjugation, hydrogenation and dehydrogenation are involved in the hepatic metabolism of CA and DHCA. The O-methylation pathway for CA and DHCA is a detoxification route whereas o-quinones formation catalyzed by P450 is the toxification route.
来源:Hazardous Substances Data Bank (HSDB)