角鲨烯 | 111-02-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−75 °C(lit.)
-
沸点:285 °C25 mm Hg(lit.)
-
密度:0.858 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
溶解度:DMSO:16.67 mg/mL(40.59 mM;需要超声波)H2O:< 0.1 mg/mL(不溶)
-
LogP:14.12 at 24℃
-
物理描述:Trans-squalene is a clear, slightly yellow liquid with a faint odor. Density 0.858 g / cm3.
-
颜色/状态:Oil; crystals from ether/methanol (-5 °C)
-
气味:Faint agreeable odor
-
蒸汽压力:6.3X10-6 mm Hg at 25 °C (est)
-
稳定性/保质期:
Stable under recommended storage conditions.
-
分解:When heated to decomposition it emits acrid smoke and irritating vapors.
-
粘度:12 cP at 25 °C
-
表面张力:33.9 mN/m at 22 °C (100 g/L)
-
折光率:Index of Refraction: 1.4990 at 20 °C
-
碰撞截面:210.9 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
-
保留指数:2808
计算性质
-
辛醇/水分配系数(LogP):11.6
-
重原子数:30
-
可旋转键数:15
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
TSCA:Yes
-
安全说明:S24/25
-
WGK Germany:2
-
海关编码:29012980
-
RTECS号:XB6010000
-
包装等级:II; III
-
危险类别:4.1
-
危险标志:GHS08
-
危险性描述:H304
-
危险性防范说明:P301 + P310,P331
SDS
制备方法与用途
反式角鲨烯是一种具有异戊二烯结构的全反式三萜烯化合物,含有6个双键,因此性质极不稳定,容易氧化。它在抑制氧化应激和清除体内的炎症因子方面表现出较好的功能特性,被广泛应用于食品、医药和化妆品等领域。
功能特性反式角鲨烯是一种强大的抗氧化物质,在体内能阻断氧化应激过程引起的生理学病变,并通过影响酶和细胞的活性,调节细胞因子、多种物质的水平及信号传递。它能够降低胆固醇合成、提高免疫系统能力、抑制肿瘤细胞生成,以及减轻外界毒性物质对机体的不良影响。
生物活性反式角鲨烯(Spinacene, Supraene)自然存在于植物、动物和人体中,是一种添加在疫苗佐剂中的成分,用于增强免疫反应。
靶点Human Endogenous Metabolite
化学性质本品是从深海鲨鱼肝或肝油中制得。它是由6个异戊二烯构成的不饱和脂肪烯烃,属于非环式的三萜结构。为无色或微黄色油状澄明液体;有特异鱼肝油萜臭。熔点-75℃,沸点240-242℃/266.644Pa,密度0.854-0.862g/cm³,折射率为1.494-1.499。能与乙醚、四氯化碳和丙酮任意混合,不溶于水。易氧化。
用途 营养药内服治疗高、低血压、贫血、糖尿病、肝硬化、癌症、便秘、虫牙;外敷治疗扁桃腺炎、喘息、支气管炎、感冒、结核、鼻炎、胃溃疡、十二指肠溃疡、胆、膀胱结石、风湿病、神经痛等。
溶剂气相色谱固定液(最高使用温度140℃,溶剂为甲苯),分离分析烃类化合物。
生产方法 方法一:以鲨鱼肝油为原料将鲨鱼肝油减压蒸馏,得3.5%的含姥鲨烷初制品。再通过硅油浴加热进行减压蒸馏,收集第I和第II馏分,最终得到精制的反式角鲨烯。
方法二:以鲸鲨肝脏为原料取新鲜冰冻的鲸鲨肝,去血水后剪碎并匀浆,经过皂化、分离等步骤获得粗制品。再通过减压蒸馏进行多次蒸馏,收集无色透明的油状物,即精制的反式角鲨烯。
方法三:以鲨肝为原料取新鲜冰冻鲸鲨肝脏,去血水后剪碎并匀浆,经过皂化、分离等步骤获得粗制品。再通过减压蒸馏进行多次蒸馏,收集无色透明的油状物,即精制的反式角鲨烯。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 反式-β-金合欢烯 (E)-β-farnesene 18794-84-8 C15H24 204.356 反式,反式-金合欢醇 Farnesol 106-28-5 C15H26O 222.371 反,反-氯化法呢酯 (2E,6E)-farnesyl chloride 67023-84-1 C15H25Cl 240.816 金合欢醇 farnesol 4602-84-0 C15H26O 222.371 反,反-法呢基溴 farnesyl bromide 28290-41-7 C15H25Br 285.267 —— Farnesal 502-67-0 C15H24O 220.355 —— farnesyl bromide 28290-41-7 C15H25Br 285.267 香叶醇 Geraniol 106-24-1 C10H18O 154.252 香叶基溴 trans-geranyl bromide 6138-90-5 C10H17Br 217.149 —— (Z)-neryl bromide 25996-10-5 C10H17Br 217.149 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (5E,9E,13E,17E)-5,9,14,18,22-pentamethyltricosa-1,5,9,13,17,21-hexaene 717114-96-0 C28H46 382.673 —— 5-(E)-2,6,10-trimethyl-1,5,9-undecatriene 62947-45-9 C14H24 192.345 —— (5E,9E)-6,10,14-trimethylpentadeca-1,5,9,13-tetraene 1322498-06-5 C18H30 246.436 —— (2E,6E,10E,14E,18E,22Z)-2,6,10,15,19-pentamethyl-tetracosa-2,6,10,14,18,22-hexaene 230637-17-9 C29H48 396.7 —— (2Z,22Z)-6,10,15,19-tetramethyl-tetracosa-2,6,10,14,18,22-hexaene —— C28H46 382.673 —— (2E,6E,10E,14E,18E,22E)-2,6,10,15,19-pentamethyl-tetracosa-2,6,10,14,18,22-hexaene 230637-18-0 C29H48 396.7 —— (E,E,E,E)-2,6,10,15,18,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene —— C30H50 410.727 —— 1-methylidenesqualene 122333-76-0 C31H50 422.738 —— (4E, 8E)-5,9,13-trimethyl-4,8,12-tetradecatrienal 67858-78-0 C17H28O 248.409 —— (4E,8E)-5,9,13-trimethyl-4,8,12-tetradecatrien-1-ol 67858-77-9 C17H30O 250.425 2,6-二甲基庚-1,5-二烯 2,6-dimethyl-hepta-1,5-diene 6709-39-3 C9H16 124.226 —— (4E,8E,12E)-4,9,13,17-Tetramethyloctadeca-4,8,12,16-tetraen-1-al 56882-09-8 C22H36O 316.527 —— (4E,8E,12E,16E)-4,8,13,17,21-pentamethyldocosa-4,8,12,16,20-pentaenal 56882-05-4 C27H44O 384.646 —— 4,8,13,17-tetramethyl-eicosa-4,8,12,16-tetraenedial 26906-73-0 C24H38O2 358.565 —— (all-E)-5,9,14,18,22-pentamethyl-5,9,13,17,21-tricosapentaen-1-yne —— C28H44 380.657 —— (all-E)-5,9,14,18-tetramethyl-5,9,13,17-docosatetraen-1,21-diyne —— C26H38 350.588 —— 1,1',2-trisnorsqualene alcohol 118599-19-2 C27H46O 386.662 —— (4E,8E,12E)-4,9,13,17-Tetramethyloctadeca-4,8,12,16-tetraen-1-ol 56882-10-1 C22H38O 318.543 —— (Z,E,E,E,E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-ol 114926-82-8 C30H50O 426.726 —— (4E,8E)-N-methyl-5,9,13-trimethyl-4,8,12-tetradecatrienylamine 142636-07-5 C18H33N 263.467 香叶基丙酮 trans geranyl acetone 3796-70-1 C13H22O 194.317 —— (2E,6E,10E,14E)-3,6,11,15,19-pentamethylicosa-2,6,10,14,18-pentaen-1-ol 784182-83-8 C25H42O 358.608 —— (2E,6E,10E,14E)-1-Bromo-3,6,11,15,19-pentamethyl-icosa-2,6,10,14,18-pentaene 784182-84-9 C25H41Br 421.505 —— (all-E)-1,1-dibromo-5,9,14,18,22-pentamethyl-1,5,9,13,17,21-tricosahexaene 118417-80-4 C28H44Br2 540.465 —— (all-E)-1,1,22,22-tetrabromo-5,9,14,18-tetramethyl-1,5,9,13,17,21-docosahexaene 118410-52-9 C26H38Br4 670.204 —— (1E,5E,9E,13E,17E)-1-(1,5,9,14,18,22-hexamethyl-1,5,9,13,17,21-tricosahexaenyl)cyclopropane —— C32H52 436.765 —— (5E,9E,13E)-5,10,14,18-tetramethylnonadeca-5,9,13,17-tetraen-2-one 784182-81-6 C23H38O 330.554 —— (5E,9E,13E)-5,10,14,18-tetramethylnonadeca-5,9,13,17-tetraen-2-ol 784182-80-5 C23H40O 332.57 —— (2E,6E,10E,14E)-6,11,15,19-tetramethyl-2-(4-methylpent-3-enyl)icosa-2,6,10,14,18-pentaen-1-ol 137372-78-2 C30H50O 426.726 —— (2Z,6E,10E,14E)-6,11,15,19-Tetramethyl-2-(4-methyl-pent-3-enyl)-icosa-2,6,10,14,18-pentaen-1-ol 137372-78-2 C30H50O 426.726 —— 2-aza-2,3-dihydrosqualene 86699-73-2 C29H51N 413.731 —— N-methylbis<(4E,8E)-5,9,13-trimethyl-4,8,12-tetradecatrienyl>amine 142636-09-7 C35H61N 495.876 4,8,13,17,21-五甲基-4,8,12,16,20-二十二碳五烯酸 turbinaric acid 56882-00-9 C27H44O2 400.645 —— (R)-12-Hydroxysqualene —— C30H50O 426.726 —— (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-6,10,14,18,22-pentaen-2-ol 14031-38-0 C30H52O 428.742 —— squalene diethylamine: N,N-diethyl-4,8,13,17,21-pentamethyl-4,8,12,16,20-docosapentaenylamine (all E) 86699-75-4 C31H55N 441.784 —— squalene bis-diethylamine: 3,24-diethyl-7,11,16,20-tetramethyl-3,24-diaza-7,11,15,19-hexacosatetraene (all E) 101185-90-4 C32H60N2 472.842 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:固定在原始埃洛石上的镍纳米颗粒:一种出色的氢化过程催化剂摘要:一种可持续的纳米材料:纳米粘土(埃洛石)和第一排过渡金属(镍)之间的合作产生了一种多功能催化剂,允许通过加氢过程合成目标产品,同时具有显着的选择性和高效的催化剂回收。DOI:10.1002/cctc.202200775
-
作为产物:描述:参考文献:名称:有机合成中的三氟甲磺酸三甲硅烷基酯1摘要:三氟甲磺酸三甲基甲硅烷基酯是有机化合物的强大甲硅烷基化剂,并充当催化剂,可加速非质子介质中的各种亲核反应。反应通过甲硅烷基与杂官能团的单中心亲电配位进行,并表现出独特的选择性。DOI:10.1016/s0040-4020(01)93263-6
-
作为试剂:参考文献:名称:Nakasaki, Nippon Kagaku Zasshi, 1953, vol. 74, p. 518摘要:DOI:
文献信息
-
Ti/Pd Bimetallic Systems for the Efficient Allylation of Carbonyl Compounds and Homocoupling Reactions作者:Alba Millán、Araceli G. Campaña、Btissam Bazdi、Delia Miguel、Luis Álvarez de Cienfuegos、Antonio M. Echavarren、Juan M. CuervaDOI:10.1002/chem.201003315日期:2011.3.28The allylation, crotylation and prenylation of aldehydes and ketones with stable and easily handled allylic carbonates is promoted by a Ti/Pd catalytic system. This Ti/Pd bimetallic system is especially convenient for the allylation of ketones, which are infrequent substrates in other related protocols, and can be carried out intramolecularly to yield five‐ and six‐membered cyclic products with good
-
Synthese von Squalen aus natürlichem und synthetischem Nerolidol作者:O. Isler、R. Rüegg、L. Chopard-dit-Jean、H. Wagner、Karl BernhardDOI:10.1002/hlca.19560390334日期:——Squalene was synthesized in a modified procedure according to P. Karrer & A. Helfensteiqa starting from naturel and from synthetic nerolidol. After purification through the thio-urea adduct the synthetic products were found to be identical with pure natural squalene in their chemical and physical properties as well as in their biological behaviour.
-
Ring Opening of Oxiranes by Trimethylsilyl Trifluoromethanesulfonate作者:Sizuaki Murata、Masaaki Suzuki、Ryoji NoyoriDOI:10.1246/bcsj.55.247日期:1982.1Trimethylsilyl trifluoromethanesulfonate promotes ring opening reactions of oxirane derivatives. The reaction course is highly affected by the structures and substitution pattern of the substrates. Tetra-, tri-, and 2,2-disubstituted oxiranes and simple cycloalkene oxides are converted to the corresponding allylic alcohol trimethylsilyl ethers. The overall transformation is interpreted in terms ofTrimethylsilyl trifluoromethanesulfonate 促进环氧乙烷衍生物的开环反应。反应过程受底物结构和取代模式的影响很大。四-、三-和2,2-二取代的环氧乙烷和简单的环烯氧化物被转化为相应的烯丙醇三甲基甲硅烷基醚。总体转化解释为三氟甲磺酸甲硅烷基酯反式加成到环氧乙烷环上,然后是碱促进的三氟甲磺酸元素的反消除。2,3-二烷基-或单烷基环氧乙烷分别异构化为相应的酮和醛。(Z)-氧化环辛烯经历跨环反应生成内-顺-2-三甲基甲硅烷氧基双环[3.3.0]辛烷。6-methyl-5-hepten-2-one 氧化物反应生成 2,2, 6-trimethyl-3-trimethylsiloxy-3,4-dihydro-2H-pyran。1,2-甲基迁移发生在 (E)-3α-t-丁基二甲基甲硅烷氧基-5α-孕烯 17α,20-氧化物反应中,得到 3α-t-丁基二甲基...
-
Selective Deoxygenation of Allylic Alcohol: Stereocontrolled Synthesis of Lavandulol作者:Hee Jin Kim、Liang Su、Heejung Jung、Sangho KooDOI:10.1021/ol200779y日期:2011.5.20Selective deoxygenation of allylic alcohol can be successfully carried out by the formation of alkoxyalkyl ether (EE or MOM), followed by Pd(dppe)Cl2-catalyzed reduction with LiBHEt3. (+)-S-Lavandulol has been efficiently synthesized by the application of this protocol to the diol derived from the Pb(OAc)4-promoted oxidative ring-opening of (−)-R-carvone. This deoxygenation method is general and selective
-
Inhibitory Activity of 8-Azadecalin Derivatives towards 2,3-Oxidosqualene: Lanosterol Cyclases from Baker’s Yeast and Pig’s Liver作者:Tsutomu Hoshino、Naoto Kobayashi、Eiichi Ishibashi、Shuji HashimotoDOI:10.1271/bbb.59.602日期:1995.18-azadecalin derivatives. Strong inhibitory activity was observed for those compounds having carbon chains around C12. Interestingly, the amide compounds (not the carbocationic intermediate) exhibited remarkably strong inhibition toward the liver cyclase, whereas they had an insignificant effect on the yeast cyclase (about 10(2)-fold less active). The yeast cyclase needed the amine functionality (carbocationic通过猪肝和贝克酵母的对比研究,研究了2,3-氧化角鲨烯:羊毛甾醇环化酶的抑制剂。抑制剂的基本骨架是8-azadecalin。通过与NaCNBH 3的还原胺化反应,将异戊二烯样链[神经节丙酮(Z-形式),香叶基丙酮(E-形式)或其氢化形式]连接到氮原子上。在这三种形式中,Z-异构体是对猪肝脏和酵母环化酶最有效的抑制剂。为了检查碳链长度(亲脂性)的影响,将各种脂肪酸(C6-C18)附加到8-azadecalin衍生物上。对于在C12附近具有碳链的那些化合物,观察到强的抑制活性。有趣的是 酰胺化合物(不是碳正离子中间体)对肝脏环化酶表现出显着的抑制作用,而对酵母环化酶的作用却微不足道(活性降低约10(2)倍)。酵母环化酶需要胺官能团(碳酸盐中间体),该胺官能团是通过使用LiAlH4从相应的酰胺中制备的,以表现出有效的抑制作用。我们发现,在任何已知物质中,N-十二烷基-8-氮杂-4,4,10β-三
表征谱图
-
氢谱1HNMR
-
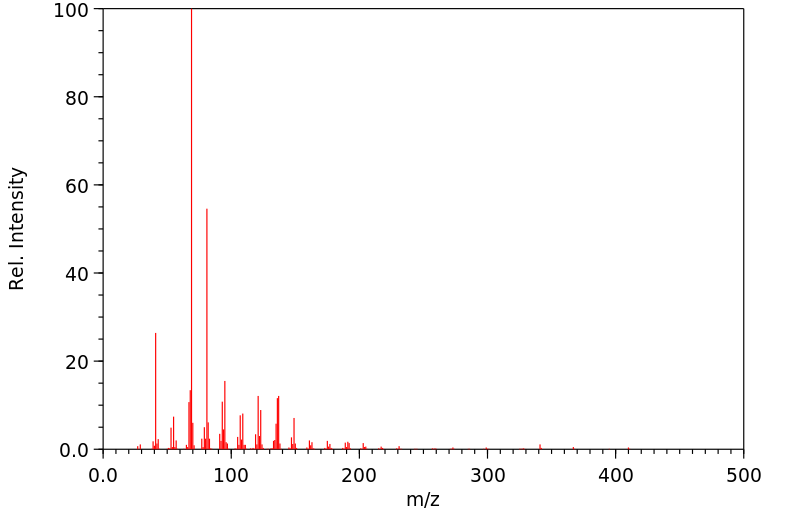
质谱MS
-
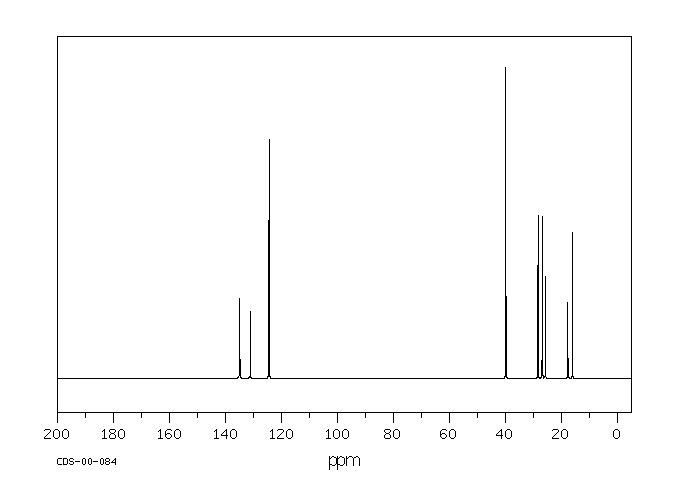
碳谱13CNMR
-
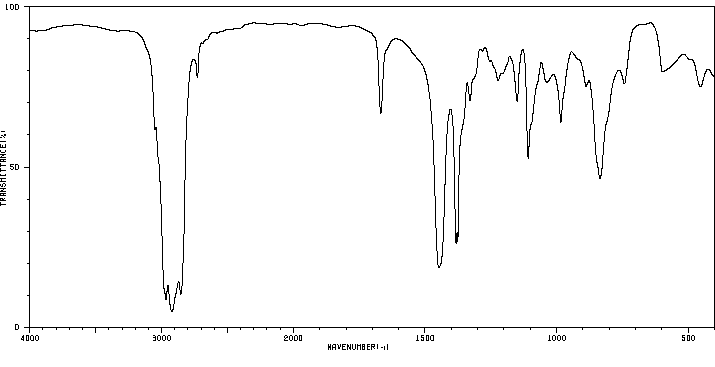
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息