代谢
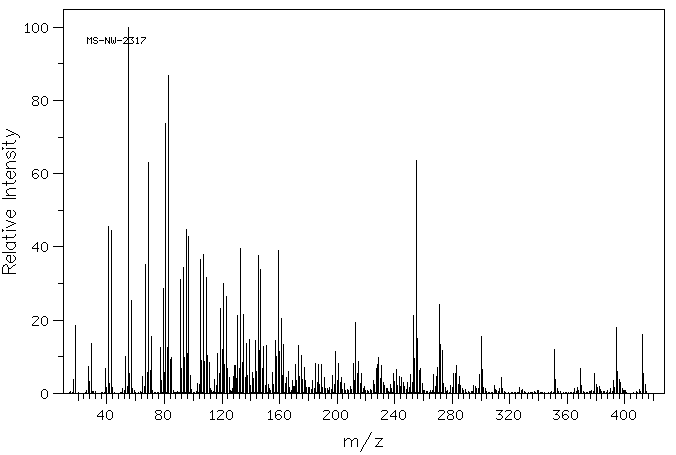
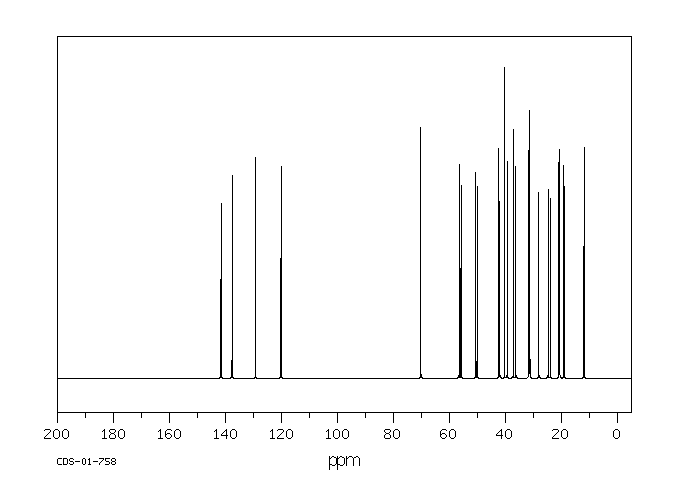
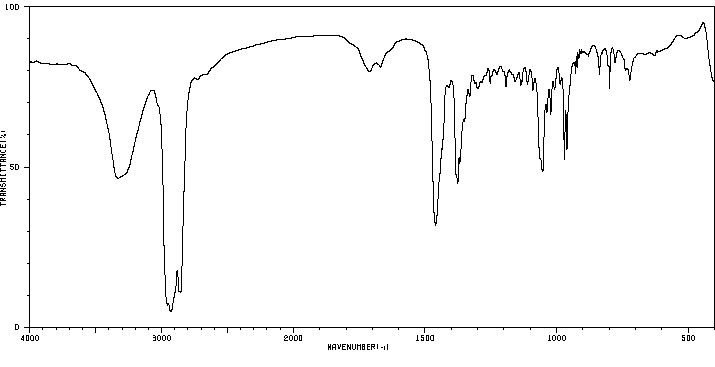
植物甾醇的代谢是通过使用大鼠粪便和肝脏微粒体来研究的。在给大鼠口服植物甾醇(一种特征明确的混合物,包含40%的β-谷固醇、30%的菜油固醇和20%的δ-谷固醇)后,收集了粪便(剂量为0.5克/千克)。使用气相色谱/质谱(GC/MS)鉴定植物甾醇的代谢物。三个峰在12.47、12.65、12.87分钟的保留时间被洗脱,并分别具有特征性的分子离子m/z 428、430、432。三种粪便代谢物被鉴定为雄烯二酮、雄烯二酮和雄烷二酮。在大鼠肝脏微粒体反应混合物中没有检测到代谢物。结果表明,在大鼠大肠中,植物甾醇的代谢物是通过3-位的氧化、5-和6-位的饱和以及17-侧链的断裂形成的。
Metabolism of phytosterols was investigated using rat feces and liver microsomes. Feces were collected after phytosterols (a well characterized mixture of beta-sitosterol 40%, campesterol 30% and dihydrobrasicasterol) were administered orally (0.5 g/kg) to rats. Metabolites of phytosterols were identified using GC/MS. Three peaks were eluted at 12.47, 12.65, 12.87 min and had characteristic molecular ions m/z 428, 430, 432, respectively. Three fecal metabolites were identified as androstadienedione, androstenedione, and androstanedione. No metabolites could be detected in the rat liver microsomal reaction mixture. The results suggest that the metabolites of phytosterols in rat feces are formed by oxidation at 3- position, saturation at 5- and 6- position, and 17- side chain cleavage in the rat large intestine.
来源:Hazardous Substances Data Bank (HSDB)