胆固醇醋酸酯 | 604-35-3
中文名称
胆固醇醋酸酯
中文别名
乙酸胆甾醇脂;胆甾-5-烯-3-醇(3β)乙酸酯;胆甾醇乙酸脂;胆固醇乙酸酯;胆甾醇乙酸酯;乙酸胆固醇酯;醋酸胆固醇酯
英文名称
Cholesteryl acetate
英文别名
cholesterol acetate;[(3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate
CAS
604-35-3
化学式
C29H48O2
mdl
——
分子量
428.699
InChiKey
XUGISPSHIFXEHZ-VEVYEIKRSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112-114 °C (lit.)
-
比旋光度:-44 º (c=2, CHCl3)
-
沸点:483.49°C (rough estimate)
-
密度:0.9935 (rough estimate)
-
溶解度:不溶的
-
LogP:10.542 (est)
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):9.1
-
重原子数:31
-
可旋转键数:7
-
环数:4.0
-
sp3杂化的碳原子比例:0.9
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
危险品运输编号:OTH
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— cholesta-5,22-dien-3β-ol acetate —— C29H46O2 426.683 醋酸豆甾醇 stigmasterol acetate 4651-48-3 C31H50O2 454.737 25-羟基胆甾-5-烯-3beta-基乙酸酯 25-hydroxycholesteryl acetate 10525-22-1 C29H48O3 444.698 胆固醇乙醚 3-ethylcholesterol 986-19-6 C29H50O 414.715 胆固醇甲基醚 cholesteryl methyl ether 1174-92-1 C28H48O 400.689 —— 3β-Vinyloxy-Δ5-cholestene —— C29H48O 412.7 —— cholester-3β-yl thioacetate 57701-06-1 C29H48OS 444.766 胆固醇 cholesterol 57-88-5 C27H46O 386.662 胆甾-5-烯-3-醇 cholesterol 765935-41-9 C27H46O 386.662 豆甾醇 Stigmasterol 83-48-7 C29H48O 412.7 —— 3-O-(tetrahydro-2H-pyran-2-yl)cholesterol 6252-45-5 C32H54O2 470.78 —— benzyl 3-cholesteryl ether 7278-60-6 C34H52O 476.786 O-三甲基硅烷基胆固醇 cholesterol trimethylsilyl ether 1856-05-9 C30H54OSi 458.844 —— cholesteryl hydrogenphosphite 66979-52-0 C27H47O3P 450.642 —— 3-[(1,1-dimethylethyl)dimethylsilyl]oxycholest-5-ene 57711-50-9 C33H60OSi 500.924 5-胆甾烯 cholest-5-ene 570-74-1 C27H46 370.662 (20S)-21-羟基-20-甲基孕甾-4-烯-3-酮 (20S)-20-hydroxymethylpregn-4-en-3-one 40736-33-2 C22H34O2 330.511 —— 3-acetoxy-cholesta-3,5-diene 2309-32-2 C29H46O2 426.683 二去甲胆烯醛 (20S)-3-oxopregn-4-ene-20-carboxaldehyde 3986-89-8 C22H32O2 328.495 —— 3-chloro-cholest-5-ene 910-31-6 C27H45Cl 405.107 氯化胆固醇 cholesteryl chloride 910-31-6 C27H45Cl 405.107 - 1
- 2
- 3
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 25-羟基胆甾-5-烯-3beta-基乙酸酯 25-hydroxycholesteryl acetate 10525-22-1 C29H48O3 444.698 胆甾烯基丙酸酯 cholesteryl propionate 633-31-8 C30H50O2 442.726 —— 3β-acetoxy-5-cholenic acid 19462-13-6 C26H40O4 416.601 (3beta)-胆甾-5-烯-3-基二十烷酸酯 20:0-cholestrol 2573-03-7 C47H84O2 681.183 —— methyl 3β-acetoxychol-5-en-24-oate 31823-53-7 C27H42O4 430.628 —— 3β-acetoxy-cholest-5-en-24-one 20981-59-3 C29H46O3 442.682 胆固醇花生四烯酸酯 cholesteryl 5,8,11,14-eicosatetraenoate 604-34-2 C47H76O2 673.119 —— [(3S,8R,9S,10R,13R,14S,17R)-13-methyl-17-[(2R)-6-methylheptan-2-yl]-1,2,3,4,7,8,9,10,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-3-yl] nonanoate 99677-89-1 C35H60O2 512.86 胆固醇乙醚 3-ethylcholesterol 986-19-6 C29H50O 414.715 —— 3β-acetoxycholest-5-en-19-ol 750-59-4 C29H48O3 444.698 孕烯醇酮醋酸酯 Pregnenolone acetate 1778-02-5 C23H34O3 358.521 (3b)-(3'b)-3,3'-氧基二-胆甾-5-烯 Dicholesterylether 2469-23-0 C54H90O 755.308 —— 3β-acetoxy-7β-hydroxycholest-5-ene 17974-77-5 C29H48O3 444.698 —— 7α-hydroxycholest-5-en-3β-yl 3-acetate 19317-90-9 C29H48O3 444.698 胆固醇甲基醚 cholesteryl methyl ether 1174-92-1 C28H48O 400.689 醋酸去氢表雄酮 prasterone acetate 853-23-6 C21H30O3 330.467 —— 3β-Acetoxy-5-cholesten-19-al 1107-90-0 C29H46O3 442.682 —— cholest-5-ene-3β,19-diol diacetate 21072-68-4 C31H50O4 486.736 —— cholester-3β-yl thioacetate 57701-06-1 C29H48OS 444.766 7-脱氢醋酸胆固醇 7-Dehydrocholesteryl acetate 1059-86-5 C29H46O2 426.683 7-氧代胆甾-5-烯-3-beta-基乙酸酯 7-ketocholesteryl acetate 809-51-8 C29H46O3 442.682 —— 7-ketopregnenolone acetate 6748-09-0 C23H32O4 372.505 (3b)-3-羟基-胆-5-烯-24-酸甲酯 methyl 5-cholenoate 20231-57-6 C25H40O3 388.591 胆固醇 cholesterol 57-88-5 C27H46O 386.662 —— cholest-5-ene-3β,7α-diol diacetate 17974-76-4 C31H50O4 486.736 —— cholest-5-ene-3β,7β-diol diacetate 18099-24-6 C31H50O4 486.736 3B-羟基-D5-胆烯酸 3β-hydroxy-5-cholenoic acid 5255-17-4 C24H38O3 374.564 25-羟基胆甾醇 25-hydroxychlolesterol 2140-46-7 C27H46O2 402.661 —— 19-norcholesterol 2137-16-8 C26H44O 372.635 —— 3β-acetoxy-17-(2-carbamoylhydrazono)-5-androstene 983-28-8 C22H33N3O3 387.522 19-羟基胆固醇 3β,19-dihydroxy-5-cholestene 561-63-7 C27H46O2 402.661 —— 5β-methyl-19-norcholest-9(10)-ene-3β,6β-diol 3,6-diacetate 2572-56-7 C31H50O4 486.736 胆甾-5-烯-3,7二醇 (3S,7S,8S,9S,10R,13R,14S,17R)-17-((R)-1,5-Dimethyl-hexyl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,7-diol 566-26-7 C27H46O2 402.661 胆甾-5-烯-3,7二醇 cholest-5-en-3β,7β-diol 566-27-8 C27H46O2 402.661 —— cholest-5-ene-3β,4α-diol 34310-86-6 C27H46O2 402.661 4-Beta-羟基胆固醇 4β-hydroxycholesterol 17320-10-4 C27H46O2 402.661 —— cholest-4-ene-3β,6β-diol diacetate 2572-51-2 C31H50O4 486.736 —— cholest-5-ene-3β,4β-diyl diacetate 17320-11-5 C31H50O4 486.736 —— 4β-acetoxycholest-5-en-3β-ol —— C29H48O3 444.698 5-胆甾烯-3β-醇-7-酮 7-Oxocholesterol 566-28-9 C27H44O2 400.645 7-羟基-4-胆甾烯-3-酮 Δ4-7α-hydrocholesterol 3862-25-7 C27H44O2 400.645 —— cholest-5-en-7-ol 898809-85-3 C27H46O 386.662 7-去氢胆固醇 7-dehydrocholesterol 434-16-2 C27H44O 384.646 —— Cholest-4-en-3β,6β-diol 570-88-7 C27H46O2 402.661 —— 3β-acetoxy-cholest-4-en-6-one 104596-45-4 C29H46O3 442.682 —— 3β,4β,7α-trihydroxy-5-cholestene 219131-32-5 C27H46O3 418.66 6-硝基胆固醇乙酸酯 3β-acetoxy-6-nitrocholest-5-ene 1912-54-5 C29H47NO4 473.696 5-胆甾烯 cholest-5-ene 570-74-1 C27H46 370.662 —— 3α,4α-epoxy-5-cholesten-7-one 22799-20-8 C27H42O2 398.629 —— 1,5,7-cholestatrien-3β-ol 54604-59-0 C27H42O 382.63 —— cholest-5-ene-3β,19-diol 3-acetate 19-tosylate 21072-69-5 C36H54O5S 598.888 delta-4,6-胆甾二烯醇 cholest-4,6-diene-3-ol 14214-69-8 C27H44O 384.646 维生素 D3 vitamin D3 67-97-0 C27H44O 384.646 4-胆甾烯-3-酮 Cholest-4-en-3-one 601-57-0 C27H44O 384.646 —— (6E)-hydroximinocholest-4-en-3β-ol 110556-49-5 C27H45NO2 415.66 —— cholest-5-en-7-one 22033-90-5 C27H44O 384.646 氯化胆固醇 cholesteryl chloride 910-31-6 C27H45Cl 405.107 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:参考文献:名称:氧甾醇:合成和抗利什曼原虫活性摘要:氧化甾醇 (2-16) 是通过甾体烯丙醇的骨架重排合成的。筛选所有衍生物的抗利什曼原虫活性。化合物 3、11 和 12 显示出有效的活性。发现化合物 12 在 48 小时时毒性最小,诱导的一氧化氮 (NO) 最高。化合物 12 对脾细胞的最小毒性证实了其最佳抗无鞭毛体作用和 NO 诱导。DOI:10.1016/j.steroids.2015.12.020
-
作为产物:描述:参考文献:名称:Kocor et al., Bulletin de l'Academie Polonaise des Sciences, Serie des Sciences Chimiques, 1958, vol. 6, p. 1,2摘要:DOI:
-
作为试剂:参考文献:名称:Rhodium acetate-catalyzed aerobic Mukaiyama epoxidation of alkenes摘要:Mukaiyama epoxidation of alkenes under oxygen catalyzed by rhodium acetate with isobutyraldehyde as the reducing agent is as or more effective than previously reported procedures. A variety of alkenes, including terpenes and cholesterol derivatives, were oxidized. And high regioselectivity for mono-epoxidation was observed with neryl, geranyl, and linalyl acetates. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2013.09.062
文献信息
-
Additives and products including oligoesters申请人:——公开号:US20030199593A1公开(公告)日:2003-10-23The present invention relates to oligoesters and their use or the creation of additives. Oligoester containing additives and/or oligoesters themselves may be used for formulating pharmaceutical preparations, cosmetics or personal care products such as shampoos and conditioners. These oligoesters are particularly useful for the creation of multi-purpose additives that can impart conditioning, long substantivity and/or UV protection. Individual oligoesters and oligoester mixtures are described.本发明涉及寡酯及其用途或添加剂的制备。含有寡酯的添加剂和/或寡酯本身可用于配制药物制剂、化妆品或个人护理产品,如洗发水和护发素。这些寡酯对于制备能够赋予调理、长效性和/或紫外线保护的多功能添加剂特别有用。描述了单独的寡酯和寡酯混合物。
-
Studies of the synthesis of 5-hydroxy 6-keto steroids and related 6-keto steroids作者:Peter Yates、Shirley StiverDOI:10.1139/v87-369日期:1987.9.1
Syntheses of 5-hydroxy-5α- and 5β-cholestan-6-one (11 and 13) and their 3β-acetoxy (10 and 21) and 3β-benzyloxy derivatives (12 and 19) are described, as are syntheses of the 7α-deutero derivatives of 10 and 21. Related investigations of the syntheses of the 5-methoxy and 5-methyl analogues of these compounds are also discussed. Treatment of 12 with potassium tert-butoxide has been shown to give 5-hydroxy-5β-cholest-3-en-6-one (14) and its Δ2 isomer 15. Reaction of 6-nitrocholesteryl acetate (50) with lithium dimethylcuprate gives 3α,5-cyclo-5α-cholestan-6-one (E)-oxime (51) as the major product.
-
3-乙酰基-5-羟基-B-降胆甾醇-6-(N-甲基)缩氨硫腙、制备方法及其用途
-
Copper-Catalyzed Intermolecular Trifluoromethylazidation of Alkenes: Convenient Access to CF<sub>3</sub>-Containing Alkyl Azides作者:Fei Wang、Xiaoxu Qi、Zhaoli Liang、Pinhong Chen、Guosheng LiuDOI:10.1002/anie.201309991日期:2014.2.10A novel copper‐catalyzed intermolecular trifluoromethylazidation of alkenes has been developed under mild reaction conditions. A variety of CF3‐containing organoazides were directly synthesized from a wide range of olefins, including activated and unactivated alkenes, and the resulting products can be easily transformed into the corresponding CF3‐containing amine derivatives.
-
Diazepinium perchlorate: a neutral catalyst for mild, solvent-free acetylation of carbohydrates and other substances作者:Santosh Kumar Giri、Rajesh Gour、K. P. Ravindranathan KarthaDOI:10.1039/c6ra28882k日期:——protecting groups such as TBDMS/TBDPS/Tr ethers and isopropylidene/benzylidene acetals present on a substrate unaffected. Regioselective hydroxyl protection in partially protected carbohydrate derivatives/polyhydroxylic compounds was possible and was proved to be a convenient time-saving alternative to the conventional synthesis of such compounds. Easy preparation of the catalyst, mild reaction conditions
表征谱图
-
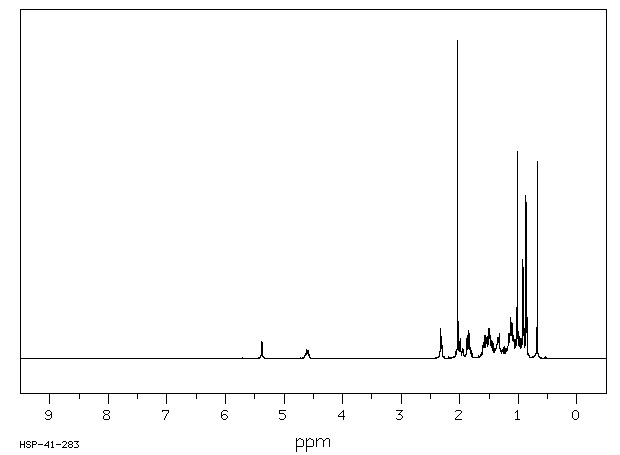
氢谱1HNMR
-
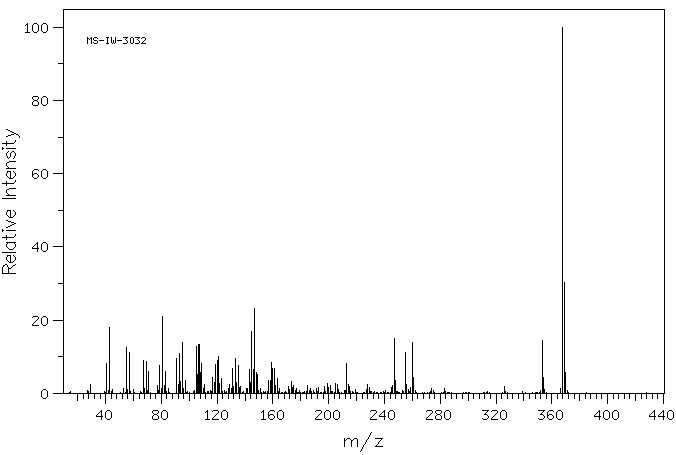
质谱MS
-
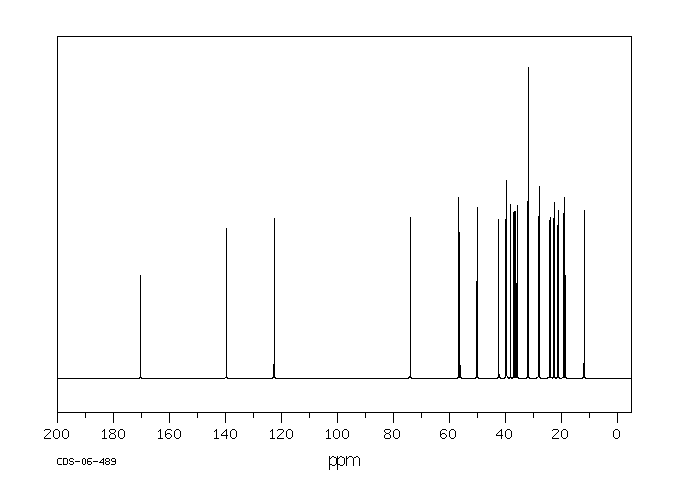
碳谱13CNMR
-
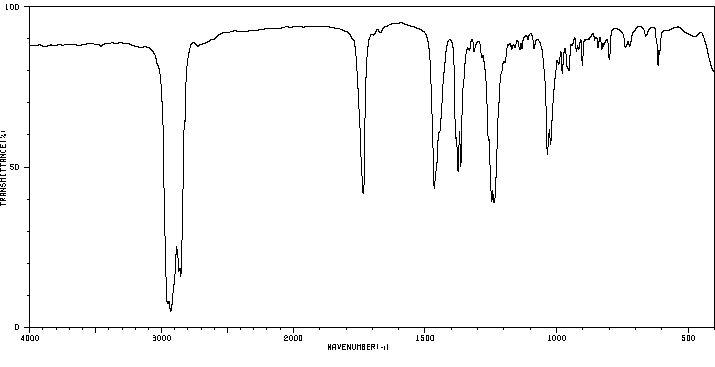
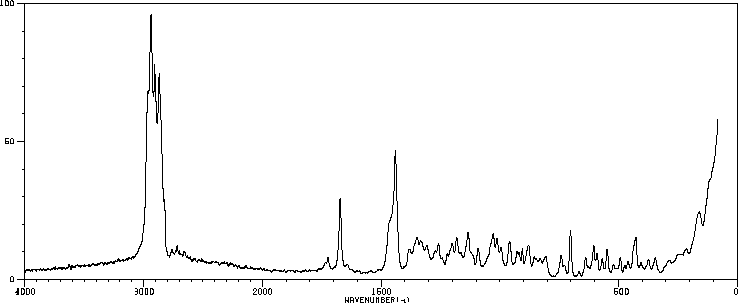
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B