芦丁 | 153-18-4
中文名称
芦丁
中文别名
路通;络通;紫皮甙;芸香叶苷;芦丁三水合物;芸香苷;维生素P;紫皮甙三水合物;路丁;维生素P三水合物;芦丁芸香苷;芸香甙;路丁粉
英文名称
rutin
英文别名
quercetin-3-rutinoside;quercetin-3-O-rutinoside;rutoside;quercitin-3-O-rutinoside;quercetin-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside;2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one;quercetin-3-O-α-rhamnopyranosyl (1'''→6'')-glucopyranoside;quercetin-3-O-β-D-glucorhamnoside;quercetin 3-O-(6-O-rhamnosylglucoside);quercetin-3-o-rutinose;quercetin rutinoside;RUT
CAS
153-18-4;1340-08-5
化学式
C27H30O16
mdl
——
分子量
610.526
InChiKey
IKGXIBQEEMLURG-NVPNHPEKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:195 °C (dec.)(lit.)
-
比旋光度:D23 +13.82° (ethanol); D23 -39.43° (pyridine)
-
沸点:576.13°C (rough estimate)
-
密度:1.3881 (rough estimate)
-
溶解度:吡啶中的溶解度为50mg/mL
-
LogP:-2.020 (est)
-
物理描述:Solid
-
碰撞截面:232.6 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):-1.3
-
重原子数:43
-
可旋转键数:6
-
环数:5.0
-
sp3杂化的碳原子比例:0.44
-
拓扑面积:266
-
氢给体数:10
-
氢受体数:16
安全信息
-
危险品标志:Xn,Xi
-
安全说明:S24/25
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:2932999099
-
危险品运输编号:20kgs
-
RTECS号:VM2975000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319
SDS
制备方法与用途
芦丁概述
来源与制法
芦丁(Rutin)是一种重要的天然黄酮类化合物。主要存在于芸香科植物芸香的全草挥发油、豆科植物槐的果实(槐角)、金丝桃科植物红旱莲的全草,鼠李科植物野梧桐的叶以及蓼科植物荞麦的籽苗中。
制法芦丁可以从原料(如槐花蕾)经粉碎后用热乙醇萃取,再经过多次结晶精制而得到。具体的提取步骤包括浸提物浓缩后去除其他可溶性色素等不纯物,并通过多步结晶和活性炭精制获得高纯度的芦丁成品。
生理作用芦丁具有多种生理活性:作为维生素P的一种形式,它是一种氢传递体,参与体内氧化还原酶的作用;增强维生素C的作用并促进其在体内的蓄积。此外,芦丁还能维持血管弹性、降低通透性和脆性,并抑制透明质酸酶水解,从而增强毛细血管的抵抗力。
生理活性- 促进细胞增生,防止血细胞凝集。
- 利尿、镇咳作用。
- 抗炎和抗过敏效果。
- 降血脂、降压及保护溃疡面的作用。
芦丁难溶于冷水但可溶于热水、甲醇、乙醇以及吡啶中,并且易溶于碱性水溶液。其主要应用包括作为晶状体醛糖还原酶抑制剂、抗炎和抗病毒作用等。
详细用途- 食用色素:具有柠檬黄色调,可用于食品着色。
- 抗氧化剂及营养补充剂:用于预防或治疗与毛细血管脆弱性相关的疾病。
- 药物:防治高血压脑出血、糖尿病视网膜病变和紫癜性疾病等。
芦丁的生产可通过从槐花米中提取获得。具体步骤包括用热水和石灰乳煮沸,添加硼砂并保温过滤后调至pH值适宜进行结晶析出,并经过多步精制工艺得到成品。对于原料(一般为槐花蕾),也可通过乙醇萃取、乙醚、热甲醇及热水多次结晶精制的方法来获取高纯度的芦丁。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异槲皮苷 isoquercetin 482-35-9 C21H20O12 464.383 —— quercetin 3-O-<β-D-apiofuranosyl(1->2)α-L-rhamnopyranosyl(1->6)>-β-D-glucopyranoside) —— C32H38O20 742.642 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 6-O-{6-deoxy-2-O-[(4-hydroxy-3,5-dimethoxyphenyl)carbonyl]-α-L-mannopyranosyl}-β-D-glucopyranoside —— C36H38O19 774.686 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— quercetin-3'-O-methyl-3-O-α-L-rhamnopyranosyl(1->6)-β-D-glucopyranoside —— C28H32O16 624.552 —— rhamnetin 3-O-rutinoside 34202-83-0 C28H32O16 624.552 —— 7-mono-O-(β-hydroxyetyl)rutoside 23869-24-1 C29H34O17 654.579 —— 7,4′-di-O-methylquercetin-3-O-beta-rutinoside 20188-85-6 C29H34O16 638.579 异槲皮苷 isoquercetin 482-35-9 C21H20O12 464.383 4',7-双(Β-羟乙基)芸香苷 7,4'-di-hydroxyethyl rutoside 13190-91-5 C31H38O18 698.632 —— quercetin-3-O-rutinoside-7-O-glucoside 30311-61-6 C33H40O21 772.668 曲克芦丁 troxerutin 7085-55-4 C33H42O19 742.685 —— Trihydroxyethylrutin —— C33H42O19 742.7 —— 4',4''',5,5'',7,7''-hexahydroxy-3,3''-O-rutinosyl-3',3'''-bisflavonoyl ether 1326303-82-5 C54H58O31 1203.04 —— 3-{{6-O-[4-O-(3-carboxy-1-oxopropyl)-6-deoxy-α-L-mannopyranosyl]-β-D-glucopyranosyl}oxy}-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one —— C31H34O19 710.6 —— 5,7,3',4'-O-tetramethylrutin 58528-03-3 C31H38O16 666.633 槲皮素 3,4'-二葡糖甙 4′-O-β-D-glucopyranosyl-quercetin-3-O-β-D-glucopyranoside 29125-80-2 C27H30O17 626.525 —— rutin palmitate 823789-36-2 C43H60O17 848.939 —— rutin laureate 917872-77-6 C39H52O17 792.832 —— 3''-O-butanoylrutin 131981-32-3 C31H36O17 680.617 异槲皮苷 isoquercitrin 21637-25-2 C21H20O12 464.383 —— 3-{{6-O-[2,3-O-(2-carboxy-1-methylidene)-6-deoxy-α-L-mannopyranosyl]-β-D-glucopyranosyl}oxy}-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one —— C31H34O18 694.6 —— 3,3′,4′,7-tetrahydroxy-5-methoxyflavone 3-O-β-D-glucuronopyranoside —— C22H22O12 478.409 —— azaleatin 3-O-β-D-galactoside —— C22H22O12 478.409 —— quercetin 3,7-O-β-D-diglucoside 6892-74-6 C27H30O17 626.525 —— 5,7,3',4'-tetra-hydroxyethyl rutoside 31511-31-6 C35H46O20 786.738 —— 7,3',4'-trihydroxyethylisoquercitrine-3-glucoside 266363-39-7 C27H32O15 596.542 —— rutin linolenate —— C45H58O17 870.945 —— 2-(3,4-dimethoxyphenyl)-5,7-dimethoxy-3-(((2S,3R,4S,5S,6R)-3,4,5-trimethoxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trimethoxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-methyl)tetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one 50991-85-0 C37H50O16 750.794 —— 6''-O-cinnamoylisoquercitrin —— C30H26O13 594.529 —— quercetin-3-O-tetra-O-acetyl-β-D-glucopyranoside 39028-59-6 C29H28O16 632.532 —— 3-O-(2'',3'',4'',6''-tetra-O-acetyl-β-D-galactopyranosyl)-3',4',5,7-tetrahydroxyflavonol 161126-00-7 C29H28O16 632.532 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{{2,3,4-tris-O-(3-carboxy-1-oxopropyl)-6-O-[2,3,4-tris-O-(3-carboxy-1-oxopropyl)-6-deoxy-α-L-mannopyranosyl]-β-D-glucopyranosyl}oxy}-4H-1-benzopyran-4-one 267006-02-0 C51H54O34 1210.97 —— quercetin-3-O-β-D-(6-O-Z-p-coumaroyl)glucopyranoside —— C30H26O14 610.528 —— quercetin 3-O-β-D-(6″-O-(E)-p-coumaryl) glucopyranoside —— C30H26O14 610.528 —— 3-O-β-D-glucopyranosyl-3',4',7-tri-O-benzyl-5-hydroxyflavonol —— C42H38O12 734.757 —— quercetin 3-O-(6″-O-E-caffeoyl)-β-D-glucopyranoside —— C30H26O15 626.527 —— 3-methoxy-5,3',4'-trihydroxy-7-O-β-D-glucosylflavone 26931-68-0 C22H22O12 478.409 —— quercetin 3-methyl ether 7-O-β-D-galactopyranoside —— C22H22O12 478.409 —— 2'',2''',3',3'',3''',4',4'',4''',5,7-deca-O-acetylrutin 2328-13-4 C47H50O26 1030.9 —— Caryatin-7-O-β-D-glucoside —— C23H24O12 492.436 —— [(2S,3R,4S,5R,6R)-2-[2-[3,4-bis(phenylmethoxy)phenyl]-4-oxo-5,7-bis(phenylmethoxy)chromen-3-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl] benzoate 1309453-94-8 C56H48O13 928.989 地奥司明 diosmin 520-27-4 C28H32O15 608.553 —— 4-(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yloxy)butanenitrile —— C19H15NO7 369.331 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-(2-hydroxyethoxy)-4H-chromen-4-one —— C17H14O8 346.293 —— 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-propoxychromen-4-one —— C18H16O7 344.321 —— quercetin-3-O-acetic acid 1144104-73-3 C17H12O9 360.277 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-isopropoxy-4H-chromen-4-one —— C18H16O7 344.321 —— 2-(3,4-bis(methoxymethoxy)phenyl)-5-hydroxy-3,7-bis(methoxymethoxy)-4H-chromen-4-one 143724-67-8 C23H26O11 478.453 —— 2-(3,4-Diethoxyphenyl)-5,7-diethoxy-3-(2-hydroxyethoxy)chromen-4-one 82547-01-1 C25H30O8 458.508 —— 2-(3,4-dihydroxyphenyl)-3-ethoxy-5,7-dihydroxy-4H-chromen-4-one —— C17H14O7 330.294 —— 5-O-prenyl-3,3',7,4'-tetrakis-O-methoxymethylquercetin 143724-71-4 C28H34O11 546.571 —— Ethyl 2-[2-(3,4-diethoxyphenyl)-5,7-diethoxy-4-oxochromen-3-yl]oxyacetate 82546-99-4 C27H32O9 500.546 —— 2-[2-(3,4-Diethoxyphenyl)-5,7-diethoxy-4-oxochromen-3-yl]oxyacetic acid 82547-00-0 C25H28O9 472.492 —— 2-((2-(3,4-dimethoxyphenyl)-5,7-dimethoxy-4-oxo-4H-chromen-3-yl)oxy)acetic acid —— C21H20O9 416.384 —— 7,4'-dihydroxy-3,5,3'-triethoxyflavone —— C21H22O7 386.401 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-ylacetate 143631-97-4 C17H12O8 344.277 —— [4-[4-oxo-3,5,7-tri(propanoyloxy)chromen-2-yl]-2-propanoyloxyphenyl] propanoate 52739-60-3 C30H30O12 582.561 —— 3',4'-O-(phenylbenzylidene)-3-O-n-propylquercetin —— C31H24O7 508.527 [5,7-二乙酰氧基-2-(3,4-二乙酰氧基苯基)-4-氧代苯并吡喃-3-基]乙酸酯 3,5,7-triacetoxy-2-(3,4-diacetoxy-phenyl)-chromen-4-one 1064-06-8 C25H20O12 512.427 3-邻甲基槲皮素 2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-methoxy-chromen-4-on 1486-70-0 C16H12O7 316.267 3',5,7-三羟基-3,4'-二甲氧基黄酮 3,4'-O-dimethylquercetin 33429-83-3 C17H14O7 330.294 5,3',4'-三羟基-3,7-二甲氧基黄酮 2-(3,4-dihydroxyphenyl)-5-hydroxy-3,7-dimethoxychromen-4-one 2068-02-2 C17H14O7 330.294 —— 3',4'-O-(phenylbenzylidene)-3-O-ethylquercetin —— C30H22O7 494.5 5,3'-二羟基-3,7,4'-三甲氧基黄酮 ayanin 572-32-7 C18H16O7 344.321 —— 7-(benzyloxy)-2-(3,4-bis(benzyloxy)phenyl)-5-hydroxy-3-methoxy-4H-chromen-4-one 1486-57-3 C37H30O7 586.641 栎精-3,5,7,3,4-五甲醚 pentamethyl quercetin 1247-97-8 C20H20O7 372.375 —— 8-prenyl-3,3',7,4'-tetrakis-O-methoxymethylquercetin 143724-77-0 C28H34O11 546.571 —— 3-(benzyloxy)-2-(3,4-dimethoxyphenyl)-5-hydroxy-7-methoxy-4H-benzopyran-4-one —— C25H22O7 434.445 —— 3,7-bis-benzyloxy-2-(4'-benzyloxy-3'-hydroxyphenyl)-5-hydroxy-chromen-4-one 40554-92-5 C36H28O7 572.614 —— 2-(2,2-diphenylbenzo[1,3]dioxol-5-yl)-5,7-dihydroxy-3-methoxychromen-4-one —— C29H20O7 480.474 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-fluorobenzoate —— C22H13FO8 424.339 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 2-fluorobenzoate —— C22H13FO8 424.339 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl benzoate —— C22H14O8 406.348 —— 3-O-[(3″,4″,5″-tri-O-methoxymethyl)-galloyl]quercetin 1420045-11-9 C28H26O14 586.506 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 3,4,5-trihydroxybenzoate 700364-44-9 C22H14O11 454.347 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 3-fluorobenzoate —— C22H13FO8 424.339 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-aminobenzoate —— C22H15NO8 421.363 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-methoxybenzoate —— C23H16O9 436.375 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 2-aminobenzoate —— C22H15NO8 421.363 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 3-methoxybenzoate —— C23H16O9 436.375 —— 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 2-methoxybenzoate —— C23H16O9 436.375 —— [2-[3,4-Bis(phenylmethoxy)phenyl]-4-oxo-5,7-bis(phenylmethoxy)chromen-3-yl] 3,4,5-tris(methoxymethoxy)benzoate 1420045-10-8 C56H50O14 947.005 —— 3,5-Dihydroxy-3',4',7-tri-(β-hydroxyethoxy)flavon 23077-88-5 C21H22O10 434.4 —— quercetin pentabenzoate 120036-90-0 C50H30O12 822.781 —— Tetraethyl-5,7,3',4'quercetine 82546-98-3 C23H26O7 414.455 3-羟基-3,4,5,7-四甲氧基黄酮 2-(3,4-dimethoxyphenyl)-3-hydroxy-5,7-dimethoxy-4H-4-chromenone 1244-78-6 C19H18O7 358.348 —— 7-(benzyloxy)-2-(3,4-bis(benzyloxy)phenyl)-3,5-dihydroxy-4H-chromen-4-one 183067-65-4 C36H28O7 572.614 3,4,7-三甲氧基槲皮素 2-(3,4-dimethoxyphenyl)-3,5-dihydroxy-7-methoxy-4H-chromen-4-one 6068-80-0 C18H16O7 344.321 —— 5,7-bis(benzyloxy)-2-(3,4-bis(benzyloxy)phenyl)-3-hydroxy-4H-chromen-4-one 116973-12-7 C43H34O7 662.739 鼠李素 rhamnetin 90-19-7 C16H12O7 316.267 3,5,3'-三羟基-7,4'-二甲氧基黄酮 ombuin 529-40-8 C17H14O7 330.294 柽柳黄素 tamarixetin 603-61-2 C16H12O7 316.267 —— 4',7-di-O-benzylquercetin 13459-13-7 C29H22O7 482.489 —— 2-[3-[2,6-Dinitro-4-(trifluoromethyl)phenoxy]-4-hydroxyphenyl]-3,5,7-trihydroxychromen-4-one 1333241-01-2 C22H11F3N2O11 536.331 —— [2-[3,4-Bis(phenylmethoxy)phenyl]-5-hydroxy-4-oxo-7-phenylmethoxychromen-3-yl] methanesulfonate 879217-58-0 C37H30O9S 650.706 槲皮素 quercetol 117-39-5 C15H10O7 302.24 —— 8-Prenylquercetin 143724-75-8 C20H18O7 370.359 —— 3-(Benzylamino)-2-[3,4-bis(phenylmethoxy)phenyl]-5-hydroxy-7-phenylmethoxychromen-4-one 879217-59-1 C43H35NO6 661.754 3,4,5,7-四甲氧基黄酮 2-(3,4-dimethoxyphenyl)-5,7-dimethoxy-4H-chromen-4-one 855-97-0 C19H18O6 342.348 —— 3-aminoluteorin —— C15H11NO6 301.255 7,3',4'-三-O-甲基毛地黄黄酮 5-hydroxy-3',4',7-trimethoxyflavone 29080-58-8 C18H16O6 328.321 木犀草素 2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on 491-70-3 C15H10O6 286.241 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:Synthesis of quercetin 3-O-β-d-apiofuranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→6)]-β-d-glucopyranoside摘要:A concise method to construct a unique 2,6-branched trisaccharide was established by regioselective glycosylation of three free hydroxyl groups on a 3-O-protected glucose moiety, and successfully used in the synthesis of quercetin 3-O-beta-D-apiofuranosyl-(1 -> 2)-[alpha-L-rhamnopyranosyl-(1 -> 6)]-beta-D-glucopyranoside, a flavonol O-glycoside isolated from glandless cotton seeds which showed notable antidepressant activities. (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2011.04.040
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 α-acetobromorutinose 作用下, 生成 芦丁参考文献:名称:Samochwalow et al., Doklady Akademii Nauk SSSR, 1958, vol. 123, p. 305摘要:DOI:
-
作为试剂:参考文献:名称:Stereoselective reversible ketone formation from 10-hydroxylated nortriptyline metabolites in human liver摘要:1. E- and Z-10-hydroxynortriptyline are major metabolites of amitriptyline and nortriptyline in man. Upon incubation with human liver microsomes or cytosol, these metabolites were oxidized to the corresponding ketones, E- and Z-10-oxonortriptyline. (+)-E- and (+)-Z-10-hydroxynortriptyline were distinctly preferred over the (-)-isomers as substrates. NADP(+) supported the oxidation in cytosol whereas in microsomes NAD(+) was the best cofactor.2. Incubation of E- and Z-10-oxonortriptyline with NADPH and cytosol resulted in the nearly exclusive formation of (+)-E- and (+)-Z-10-hydroxynortriptyline. Kinetic analysis revealed high-affinity reduction (K-m 1-2 mu M) of the two ketones and an additional low-affinity component with the E-isomer. 10-Oxonortriptyline reduction was also catalysed by rabbit, but not by rat or guinea pig liver cytosol.3. With [4-H-3]NADPH as cosubstrate, tritium was incorporated into E- and Z-10-hydroxynortriptyline preferentially from the pro-4R position. Redox cycling of (+)-E- and (+)-Z-10-hydroxynortriptyline in cytosol in the presence of NAD(+) and NADPH was indicated by H-3 incorporation from [pro-4R-H-3]NADPH.4. Recombinant human carbonyl reductase catalysed low-affinity reduction of E-10-oxonortriptyline with preferential transfer of the pro-4S-H-3 of labelled NADPH.5. Ketone reduction in cytosol was strongly inhibited by 9,10-phenanthrenequinone and dehydrolithocholic acid and moderately by other 3-oxo steroids and some antiinflammatory drugs.6. The high-affinity reduction of E- and Z-10-oxonortriptyline and the oxidation of the alcohols in cytosol are probably mediated by a member of the aldo-keto reductase family of enzymes.DOI:10.3109/00498259509061920
文献信息
-
Flavonoid derivative申请人:Buchholz Herwig公开号:US20070134172A1公开(公告)日:2007-06-14The invention relates to a novel flavonoid derivative, to an extract comprising the flavonoid derivative, to the cosmetic and pharmaceutical use thereof, to preparations comprising the flavonoid derivative or extract, and to a process for the preparation of the flavonoid derivative or extract.
-
[EN] FIBRIN-BINDING COMPOUNDS FOR IMAGING AND TREATMENT<br/>[FR] COMPOSÉS DE LIAISON À LA FIBRINE POUR IMAGERIE ET TRAITEMENT申请人:COLLAGEN MEDICAL LLC公开号:WO2021081430A1公开(公告)日:2021-04-29This disclosure relates to compounds of Formula IV: for fibrin imaging, wherein the compounds comprise an imaging or therapeutic radioisotope.这项披露涉及到公式IV的化合物:用于纤维蛋白成像,其中化合物包含成像或治疗放射性同位素。
-
NOVEL QUERCETIN DERIVATIVES AS ANTI-CANCER AGENTS申请人:Joshi Narendra Shriram公开号:US20110034413A1公开(公告)日:2011-02-10The present invention provides novel Quercetin derivatives of formula (I) and pharmaceutically acceptable salts, hydrates, and solvates thereof, wherein R 1 is hydrogen, benzyl or substituted benzyl; R 2 is hydrogen, benzyl or substituted benzyl, linear or branched (C 1 -C 6 ) alkyl, substituted alkyl, aryl, substituted aryl, heterocycle and substituted heterocycle, useful for treatment of various disorders including cancer, multi-drug resistant cancers, viral infections etc. The invention also provides a process for the preparation of compounds of formula (I) and pharmaceutical compositions comprising the same.
-
Structural Requirements of Flavonoids and Related Compounds for Aldose Reductase Inhibitory Activity.作者:Hisashi Matsuda、Toshio Morikawa、Iwao Toguchida、Masayuki YoshikawaDOI:10.1248/cpb.50.788日期:——The methanolic extracts of several natural medicines and medicinal foodstuffs were found to show an inhibitory effect on rat lens aldose reductase. In most cases, flavonoids were isolated as the active constituents by bioassay-guided separation, and among them, quercitrin (IC50=0.15 μM), guaijaverin (0.18 μM), and desmanthin-1 (0.082 μM) exhibited potent inhibitory activity. Desmanthin-1 showed the most potent activity, which was equivalent to that of a commercial synthetic aldose reductase inhibitor, epalrestat (0.072 μM). In order to clarify the structural requirements of flavonoids for aldose reductase inhibitory activity, various flavonoids and related compounds were examined. The results suggested the following structural requirements of flavonoid: 1) the flavones and flavonols having the 7-hydroxyl and/or catechol moiety at the B ring (the 3′,4′-dihydroxyl moiety) exhibit the strong activity; 2) the 5-hydroxyl moiety does not affect the activity; 3) the 3-hydroxyl and 7-O-glucosyl moieties reduce the activity; 4) the 2–3 double bond enhances the activity; 5) the flavones and flavonols having the catechol moiety at the B ring exhibit stronger activity than those having the pyrogallol moiety (the 3′,4′,5′-trihydroxyl moiety).发现,几种天然药材和药用食品的甲醇提取物对大鼠晶状体醛糖还原酶显示抑制作用。在大多数情况下,通过生物测定指导的分离方法,分离得到黄酮类化合物作为活性成分,其中,槲皮苷(IC50=0.15 μM)、愈创木脂苷(0.18 μM)和去甲基芸香糖苷-1(0.082 μM)显示出强的抑制活性。去甲基芸香糖苷-1显示了最强的活性,相当于商品化合成醛糖还原酶抑制剂依帕司他(0.072 μM)的活性。为了阐明黄酮类化合物对醛糖还原酶抑制活性的结构要求,检测了各种黄酮类化合物及相关化合物。结果表明,黄酮类化合物的以下结构要求是:1)具有7-羟基和/或邻苯二酚结构的黄酮和黄酮醇(在B环上的3′,4′-二羟基结构)显示出强的活性;2)5-羟基结构并不影响活性;3)3-羟基和7-O-葡糖基结构降低活性;4)2-3双键增强活性;5)具有邻苯二酚结构的黄酮和黄酮醇显示出比具有连苯三酚结构(3′,4′,5′-三羟基结构)的化合物更强的活性。
-
METHOD OF IMPROVING STABILITY OF SWEET ENHANCER AND COMPOSITION CONTAINING STABILIZED SWEET ENHANCER申请人:TACHDJIAN Catherine公开号:US20120041078A1公开(公告)日:2012-02-16The present invention includes methods of stabilizing one or more sweet enhancers when they are exposed to a light source as well as liquid compositions containing one or more sweet enhancers and one or more photostabilizers.本发明包括在甜味增强剂暴露于光源时稳定一个或多个甜味增强剂的方法,以及包含一个或多个甜味增强剂和一个或多个光稳定剂的液体组合物。
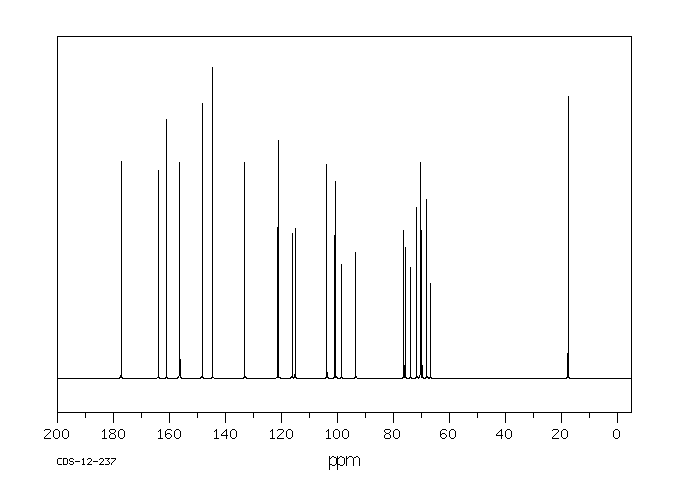
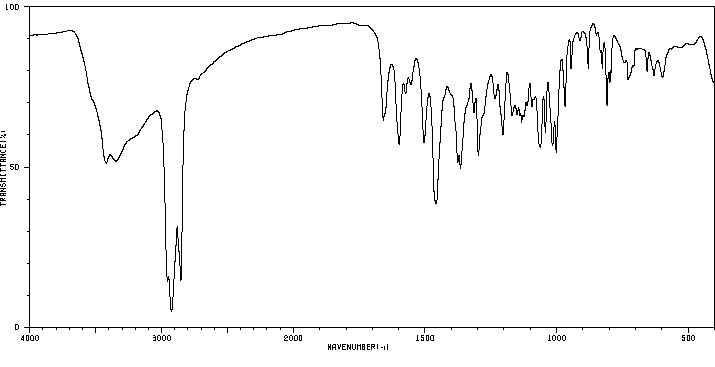
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷