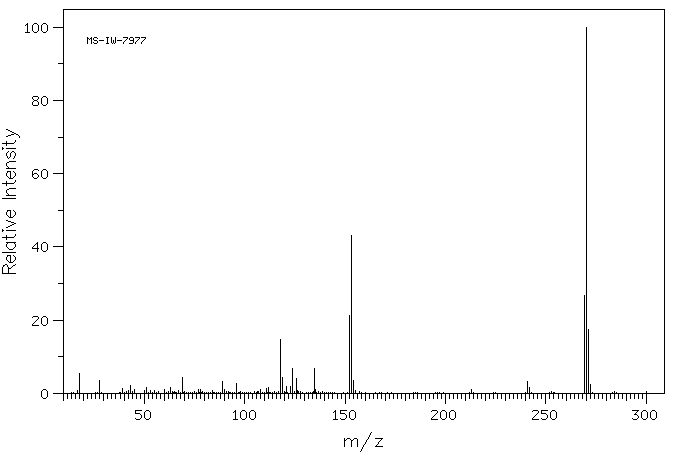
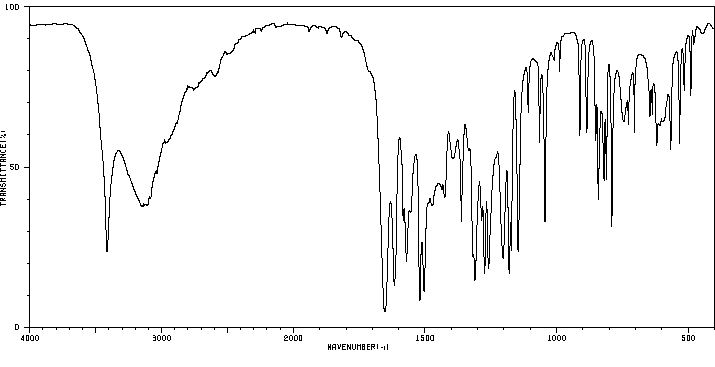
染料木素 | 446-72-0
中文名称
染料木素
中文别名
金雀异黄酮;三羟基异黄酮;5,7-二羟基-3-(4-羟苯基)-4H-1-苯并吡喃-4-酮;金雀异黄素;染料木黄酮;4',5,7-三羟基异黄酮;5,7,4'-三羟基异黄酮(Genistein);5,7,4-三羟基异黄酮
英文名称
5,7-Dihydroxy-3-(4-hydroxy-phenyl)-chromen-4-on
英文别名
genistein;Gen;5,7,4′-trihydroxyisoflavone;4',5,7-trihydroxyisoflavone;5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one;5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one;5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one;4’,5,7-trihydroxyisoflavone;5,7,4'-trihydroxyisoflavone
CAS
446-72-0
化学式
C15H10O5
mdl
MFCD00016952
分子量
270.241
InChiKey
TZBJGXHYKVUXJN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:297-298 °C
-
沸点:333.35°C (rough estimate)
-
密度:1.2319 (rough estimate)
-
溶解度:可溶于DMSO
-
LogP:3.114 (est)
-
物理描述:Solid
-
颜色/状态:Rectangular or six-sided rods from 60% alcohol. Dendritic needles from ether
-
蒸汽压力:5.2X10-12 mm Hg at 25 °C (est)
-
解离常数:pKa1 = 7.63; pKa2 = 9.67; pKa3 = 10.80 (est)
-
碰撞截面:163.3 Ų [M+H]+ [CCS Type: DT, Method: stepped-field]
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:20
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:87
-
氢给体数:3
-
氢受体数:5
ADMET
代谢
毒物动力学和人类及实验动物的代谢数据表明,金雀异黄酮能被婴儿和成人吸收进入系统性循环。金雀异黄酮主要以葡萄糖苷酸结合物的形式循环,还有一小部分以无糖苷形式循环。金雀异黄酮可以在肠道或肝脏发生葡萄糖苷酸化,但肠道在葡萄糖苷酸化过程中起着主要作用。金雀异黄酮的葡萄糖苷酸在肠肝循环过程中,可以通过肠道细菌去结合。肠道细菌在金雀异黄酮代谢中的作用已经明确建立。金雀异黄酮可以通过一条最终形成6'-羟基-O-去甲基安哥拉林(6'-hydroxy-O-demethylangolensin)的代谢途径进行代谢。一旦被吸收,金雀异黄酮的葡萄糖苷酸,以及较小程度上的金雀异黄酮无糖苷形式,会广泛分布到器官系统和胚胎。大部分金雀异黄酮剂量在24小时内通过尿液排出。
Toxicokinetic and metabolism data in humans and experimental animals indicate that genistein is absorbed into the systemic circulation of infants and adults. Genistein ... circulates as its glucuronide conjugate, and a much smaller percentage circulates as the aglycone. Genistein can be glucuronidated in the intestine or liver, but the intestine appears to play the major role in glucuronidation. Genistein glucuronides undergo enterhepatic cycling, and in the process can be deconjugated by intestinal bacteria. The role of gut bacteria in the metabolism of genistein has been clearly established. Genistein can be metabolized through a pathway that ultimately leads to the formation of 6'-hydroxy-O-demethylangolensin. Once absorbed, genistein glucuronide, and to a smaller extent genistien aglycone, are widely distributed to organ systems and the conceptus. The majority of a genistein dose is excreted in urine within 24 hours.
来源:Hazardous Substances Data Bank (HSDB)
代谢
在进入系统循环之前,大部分染料木素通过尿苷二磷酸(UDP)-葡萄糖醛酸基转移酶(UDPGT)与葡萄糖醛酸结合;还有一小部分通过磺基转移酶酶与硫酸盐结合。染料木素的结合作用发生在肠道中,尽管也有报道称在肝脏中发生。一项研究表明,催化染料木素葡萄糖醛酸化的能力以肾脏微粒体 > 结肠 > 肝脏的顺序递减。观察到的UDPGT同工酶包括1A1、1A4、1A6、1A7、1A9和1A10,它们催化染料木素的葡萄糖醛酸化。UGT 1A10同型,存在于结肠、胃和胆道上皮中,但在肝脏中不存在,被发现对染料木素有最高的活性和特异性。基于这些观察,研究作者得出结论,肠道在染料木素的葡萄糖醛酸化中起着主要作用。葡萄糖醛酸和硫酸盐结合物可以进入系统循环,循环中的大部分异黄酮化合物以结合形式存在。在研究中,当人类单独接触染料木素或与其他异黄酮苷元(以染料木素剂量的1-16 mg/kg bw计算)组合接触时,大多数染料木素以结合形式存在于血浆中;游离染料木素占总血浆染料木素水平的1-3%。结合的异黄酮经历肠肝循环,当它们返回肠道时,被具有β-葡萄糖醛酸酶或芳基硫酸酯活性的细菌去结合。这些代谢物可能被重新吸收或由肠道微生物进一步代谢。一篇综述报告称,大约10%的异黄酮在血浆中以未结合形式循环。
Prior to entering the systemic circulation, most genistein is conjugated with glucuronic acid by uridine diphosphate (UDP)-glucuronosyltransferase (UDPGT); a much smaller amount is conjugated to sulfate by sulfotransferase enzymes. Conjugation of genistein occurs in the intestine, although it also has been reported to occur in liver. One study demonstrated that the ability to catalyze glucuronidation of genistein was greatest with microsomes from kidney > colon > liver. UDPGT isoenzymes including 1A1, 1A4, 1A6, 1A7, 1A9, and 1A10 were observed to catalyze the glucuronidation of genistein. The UGT 1A10 isoform, which is present in colon, gastric, and biliary epithelium but not in liver, was observed to have the highest activity and specificity for genistein. Based on those observations, the study authors concluded that the intestine plays a major role in the glucuronidation of genistein. The glucuronide and sulfate conjugates can enter the systemic circulation, and the majority of isoflavone compounds in the circulation are present in conjugated form. In studies where humans were exposed to genistein alone or in combination with other isoflavone aglycones (calculated as genistein doses of 1-16 mg/kg bw), most of the genistein was present in plasma in conjugated form; free genistein represented 1-3% of total plasma genistein levels. The conjugated isoflavones undergo enterohepatic circulation, and upon return to the intestine, they are deconjugated by bacteria possessing beta-glucuronidase or arylsulfatase activity. The metabolites may be reabsorbed or further metabolized by gut microflora. One review reported that about 10% of isoflavonoids are circulated in plasma unconjugated.
来源:Hazardous Substances Data Bank (HSDB)
代谢
肠道微生物群对大豆食品中主要以β-糖苷结合物形式存在的异黄酮的生物转化起着关键作用。本研究使用了来自大鼠盲肠和人类粪便的微生物群来探究从大豆粉中提取的金雀异黄素β-糖苷的代谢命运。这种代谢的最终产物是通过微生物群与[2',3,5',6'-3H]和[4-14C]-标记的金雀异黄素平行孵育确定的。...通过LC-MS/IS的定量分析表明,金丝桃苷非常快速且完全降解,这与金雀异黄素的短暂增加有关。定性研究表明,金雀异黄素的马来酰和乙酰糖苷也被微生物群降解。...将盲肠和粪便微生物群与(3)H和(14)C金雀异黄素一起孵育产生了类似的放射性标记代谢物,通过放射性LC-MS(n)鉴定为中间产物二氢金雀异黄素和6'-羟基-O-去甲基芒柄花素以及最终产物4-羟基苯基-2-丙酸。金雀异黄素代谢物的这一轮廓表明,在碳原子1'和1之间选择性水解6'-羟基-O-去甲基芒柄花素,产生最终产物4-羟基苯基-2-丙酸和1,3,5-三羟基苯。...金雀异黄素代谢产物的生物学意义值得进一步研究,因为它们可能在介导食品中异黄酮消费的有益抗氧化健康效果中发挥重要作用。
Biotransformations by gut microflora play a pivotal role in determining the biological activity of isoflavones that occur in soya-based foods predominantly as betaglycosyl conjugates. Microflora prepared from rat caeca and human feces were used to investigate the metabolic fate of genistein beta-glycosides extracted from soya flour. The end-products of such metabolism were determined by parallel incubations of microflora with [2',3,5',6'-3H] and [4-14C]-labelled genistein. ... Quantitative analysis by LC-MS/IS indicated very rapid and complete degradation of genistin, which was associated with a transient increase in genistein. Qualitative studies indicated that the malonyl and acetyl glycosides of genistein were also degraded by the microflora. ... Incubation of caecal and fecal microflora with (3)H and (14)C genistein yielded similar radiolabelled metabolites, which were identified by radio-LC-MS(n) as the intermediates dihydrogenistein and 6'-hydroxy-O-desmethylangolensin and end-product 4-hydroxyphenyl-2-propionic acid. This profile of genistein metabolites indicated selective hydrolysis of 6'-hydroxy-O-desmethylangolensin between carbon atoms 1' and 1 to yield the end-products 4-hydroxyphenyl-2-propionic acid and 1,3,5-trihydroxybenzene. ... The biological significance of the products of genistein metabolism warrant further investigation since they may play an important role in mediating the beneficial antioxidant health effects associated with the consumption of isoflavones in food.
来源:Hazardous Substances Data Bank (HSDB)
代谢
植物雌激素(14-C)染料木黄酮的生物转化在雄性和雌性大鼠中进行研究,通过应用窄孔径放射性高效液相色谱-质谱(LCQ, Finnigan)来确定代谢中间体。在大鼠经灌胃给予[14C]染料木黄酮(4 mg kg(-1))24小时后,尿液中含有了五种代谢物,Gm1-Gm5。通过电喷雾电离(ESI)的结构分析揭示了分子离子(M+H)+的m/z值为447, 449, 273, 和271,分别对应于代谢物Gm2, Gm3, Gm5和染料木黄酮,以及Gm4的[M-H]-的m/z值为349。通过评估未标记和(14)C标记离子的产物离子光谱以及其对beta-葡萄糖醛酸酶处理的敏感性,推导出代谢物结构。这些研究表明代谢物与染料木黄酮葡萄糖醛酸苷(Gm2)、二氢染料木黄酮葡萄糖醛酸苷(Gm3)、染料木黄酮硫酸盐(Gm4)和二氢染料木黄酮(Gm5)是同一种物质。由于对beta-葡萄糖醛酸酶抗性的主要代谢物Gm1的ESI检测效果较差,因此通过负离子APCI进行分析;这揭示了一个去质子化的分子离子m/z 165,其色谱和质谱特性与真实的大豆酸(4-羟基苯基-2-丙酸)一致,这是染料木黄酮的一个新代谢物。在雄性和雌性大鼠的厌氧盲肠培养物中进行体外代谢研究,发现染料木黄酮通过Gm5转化为Gm1,并产生了额外的代谢物(Gm6),通过产物离子光谱鉴定为6'-羟基-O-去甲基安哥拉醇。染料木黄酮通过分离的肝细胞和精密切割的肝脏切片的生物转化主要限于母体化合物的葡萄糖醛酸化。在大鼠中发现的染料木黄酮代谢物与人类中报告的代谢物相似,这表明存在类似的生物转化途径,主要涉及肠道微生物群。
Biotransformation of the phytoestrogen (14-C)genistein was investigated in male and female rats by application of narrow-bore radio-HPLC-MSn (LCQ, Finnigan) to determine intermediates in metabolism. Urine contained five metabolites, Gm1-Gm5, 24 hr after dosing by gavage with [14C]genistein (4 mg kg(-1)). Structural analysis following ESI revealed molecular ions (M+H)+ of m/z 447, 449, 273, and 271 for metabolites Gm2, Gm3, Gm5 and genistein, respectively and an [M-H]- of m/z 349 for Gm4. Metabolite structure was deduced by evaluation of product ion spectra derived from unlabelled and (14)C-labelled ions and sensitivity to treatment with beta-glucuronidase. These studies indicated identity of metabolites with genistein glucuronide (Gm2), dihydrogenistein glucuronide (Gm3), genistein sulphate (Gm4) and dihydrogenistein (Gm5). Detection of the beta-glucuronidase resistant major metabolite Gm1 by ESI was poor and so was analysed by negative ion APCI; this revealed a deprotonated molecular ion of m/z 165 which had chromatographic and mass spectral properties consistent with authentic 4-hydroxyphenyl-2-propionic acid, a novel metabolite of genistein. In vitro metabolism studies with anaerobic caecal cultures derived from male and female rats revealed metabolism of genistein to Gm1 via Gm5 and an additional metabolite (Gm6) which was identified from product ion spectra as 6'-hydroxy-O-desmethylangolensin. Biotransformation of genistein by both isolated hepatocytes and precision-cut liver slices was limited to glucuronidation of parent compound. Commonality of genistein metabolites found in rats with those reported in man suggest similar pathways of biotransformation, primarily involving gut micro-flora.
来源:Hazardous Substances Data Bank (HSDB)
代谢
金雀异黄素在人体内已知的代谢物包括Orobol、Dihydrogenistein以及(2S,3S,4S,5R)-3,4,5-三羟基-6-[5-羟基-3-(4-羟基苯基)-4-氧杂色烯-7-基]氧杂环己烷-2-羧酸。
Genistein has known human metabolites that include Orobol, Dihydrogenistein, and (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[5-hydroxy-3-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxyoxane-2-carboxylic acid.
来源:NORMAN Suspect List Exchange
毒理性
金雀异黄素可能通过阻断细胞生长所需的酶来抑制癌细胞生长。
金雀异黄素可能通过作用于核雌激素受体来改变特定细胞基因的转录,从而降低绝经后女性的心血管风险。在随机临床试验中,观察到金雀异黄素增加了绝经后健康女性的一氧化氮与内皮素的比率,并改善了由血流介导的内皮依赖性血管舒张。此外,金雀异黄素可能通过抑制胰岛酪氨酸激酶活性以及依赖于葡萄糖和磺酰脲的胰岛素释放,对葡萄糖代谢产生有益影响。
Genistein may inhibit cancer cell growth by blocking enzymes required for cell growth.
Genistein may decrease cardiovascular risk in postmenopausal women by interacting with the nuclear estrogen receptors to alter the transcription of cell specific genes. In randomized clinical trials, genistein was seen to increase the ratio of nitric oxide to endothelin and improved flow-mediated endothelium dependent vasodilation in healthy postmenopausal women. [1] In addition, genistein may have beneficial effects on glucose metabolism by inhibiting islet tyrosine kinase activity as well as insulin release dependent on glucose and sulfonylurea. [1]
来源:Toxin and Toxin Target Database (T3DB)
毒理性
对人类不具有致癌性(未被国际癌症研究机构IARC列名)。
No indication of carcinogenicity to humans (not listed by IARC).
来源:Toxin and Toxin Target Database (T3DB)
毒理性
人类和野生动物经常暴露于混合内分泌活性化合物(EAC)。本研究的目标是调查植物雌激素染料木素对内分泌活性杀虫剂甲氧氯在生殖发育毒性方面的潜在影响。本研究使用了三种染料木素水平(0、300或800 ppm)和两种甲氧氯水平(0或800 ppm)。斯普拉格-道利大鼠在孕期和哺乳期通过饮食给母体暴露于这两种化合物,断奶后直接给后代暴露。在800 ppm时,这两种化合物,尤其是甲氧氯,与体生长减少有关,但怀孕结果并未受到任何处理的 影响。在300 ppm染料木素暴露下,观察到的影响仅限于加速雌性后代阴门开放(VO)。暴露于800 ppm染料木素或800 ppm甲氧氯会导致加速VO,并改变雌性后代动情周期的持续性。染料木素和甲氧氯共同给药的雌激素反应显然是各自单独化合物效果的累积。甲氧氯,而不是染料木素,延迟了雄性大鼠包皮分离(PPS)。当与甲氧氯一起给药时,800 ppm染料木素增强了甲氧氯对延迟PPS的效果。染料木素和甲氧氯处理并未改变两性的性别特异性运动活动模式。为了探索两种化合物在发育过程中相互作用的可能机制,我们使用染料木素和甲氧氯的主要代谢物2,2-双-(对-羟基苯基)-1,1,1-三氯乙烷(HPTE)进行了基于雌激素受体(ER)和雄激素受体(AR)的体外转录激活分析。尽管体外分析支持染料木素和甲氧氯的雌激素效果以及甲氧氯的抗雄激素效果,但这些化合物与ERα和β的反应性无法预测甲氧氯在体内比染料木素更强的雌激素效力;也无法根据体外HPTE和染料木素与AR的反应来预测染料木素增强甲氧氯对PPS的效果。来自本研究的数据表明,植物雌激素能够改变其他EAC的毒理学行为,而这些化合物的相互作用可能涉及难以根据它们的体外类固醇受体反应性预测的复杂性。
Humans and wildlife are frequently exposed to mixtures of endocrine active-compounds (EAC). The objective of the present study was to investigate the potential of the phytoestrogen genistein to influence the reproductive developmental toxicity of the endocrine-active pesticide methoxychlor. Three levels of genistein (0, 300, or 800 ppm) and two levels of methoxychlor (0 or 800 ppm) were used in this study. Sprague-Dawley rats were exposed to the two compounds, either alone or in combinations, through dietary administration to dams during pregnancy and lactation and to the offspring directly after weaning. Both compounds, methoxychlor in particular, were associated with reduced body growth at 800 ppm, but pregnancy outcome was not affected by either treatment. An acceleration of vaginal opening (VO) in the exposed female offspring was the only observed effect of genistein at 300 ppm. Exposure to 800 ppm genistein or 800 ppm methoxychlor caused accelerated VO and also altered estrous cyclicity toward persistent estrus in the female offspring. The estrogenic responses to genistein and methoxychlor administered together were apparently accumulative of the effects associated with each compound alone. Methoxychlor, but not genistein, delayed preputial separation (PPS) in the male rats. When administered with methoxychlor, genistein at 800 ppm enhanced the effect of methoxychlor on delaying PPS. Genistein and methoxychlor treatment did not change gender-specific motor activity patterns in either sex. To explore possible mechanisms for interaction between the two compounds on development, we performed estrogen receptor (ER)- and androgen receptor (AR)-based in vitro transcriptional activation assays using genistein and the primary methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE). While the in vitro assays supported the estrogenic effects of genistein and methoxychlor and the antiandrogenic effects of methoxychlor, the reactivity of these compounds with ERs alpha and beta could not predict the greater in vivo estrogenic potency of methoxychlor over genistein; nor could the potentiation of the methoxychlor effect on PPS by genistein be predicted based on in vitro HPTE and genistein reactions with the AR. Data from this study indicate that phytoestrogens are capable of altering the toxicological behaviors of other EACs, and the interactions of these compounds may involve complexities that are difficult to predict based on their in vitro steroid receptor reactivities.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
大豆异黄酮染料木素与抗雌激素他莫昔芬(TAM)对在去势裸鼠中植入的雌激素(E)依赖性乳腺癌(MCF-7)细胞生长的相互作用进行了研究。共使用了六种处理组:对照组(C);0.25毫克雌二醇(E2)植入(E);E2植入+2.5毫克TAM植入(2.5 TE);E2植入+2.5毫克TAM植入+1000 ppm染料木素(2.5 TEG);E2植入+5毫克TAM植入(5 TE),以及E2植入+5毫克TAM植入+1000 ppm染料木素(5 TEG)。使用TAM(2.5 TE和5 TE)治疗抑制了在去势裸鼠中E2刺激的MCF-7肿瘤生长。饮食中的染料木素抵消/压倒了TAM对MCF-7肿瘤生长的抑制作用,降低了血浆中的E2水平,并增加了E反应基因(例如,pS2、PR和cyclin D1)的表达。对于正在接受TAM治疗E反应性乳腺癌的绝经后妇女,在摄入染料木素时应谨慎。
... Interactions between the soy isoflavone, genistein, and an antiestrogen, tamoxifen (TAM), on the growth of estrogen (E)-dependent breast cancer (MCF-7) cells implanted in ovariectomized athymic mice /were investigated/. ... Six treatment groups were used: control (C); 0.25 mg estradiol (E2) implant (E); E2 implant + 2.5 mg TAM implant (2.5 TE); E2 implant + 2.5 mg TAM implant + 1000 ppm genistein (2.5 TEG); E2 implant + 5 mg TAM implant (5 TE), and E2 implant +5 mg TAM implant +1000 ppm genistein (5 TEG). Treatment with TAM (2.5 TE and 5 TE) suppressed E2-stimulated MCF-7 tumor growth in ovariectomized athymic mice. Dietary genistein negated/overwhelmed the inhibitory effect of TAM on MCF-7 tumor growth, lowered E2 level in plasma, and increased expression of E-responsive genes (e.g., pS2, PR, and cyclin D1). ... Caution is warranted for postmenopausal women consuming dietary genistein while on TAM therapy for E-responsive breast cancer.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
抗肿瘤药物染料木素能够抑制来自各种恶性肿瘤的肿瘤细胞株的生长。...作者们研究了染料木素与电离辐射(IR)联合治疗对宫颈HeLa细胞的疗效及其可能的作用机制。研究发现,联合处理的细胞抑制率显著高于单独使用IR或染料木素处理的细胞。在IR(4 Gy)联合染料木素(40微摩尔/升)处理后,细胞的凋亡指数显著增加,并且细胞被阻滞在G2/M期。在IR(4 Gy)处理后,Survivin mRNA的表达增加,而在联合处理后显著降低。这些结果表明,染料木素增强了宫颈癌细胞HeLa对辐射的敏感性,其作用机制可能包括增加凋亡、降低Survivin表达以及延长细胞周期阻滞。
The anticancer agent genistein inhibits cell growth of tumor cell lines from various malignancies. ... /The authors/ investigated the effectiveness of combined treatment of ionizing radiation (IR) with genistein on cervical HeLa cells and its possible mechanism. It was found that the inhibitory rate in cells with combined treatment was significantly higher than that of the cells treated with IR or genistein alone. After treatments of IR (4 Gy) combined with genistein (40 micromol/L), the apoptotic index of the cells was significantly increased and the cells were arrested in the G2/M phase. Survivin mRNA expression increased after IR (4 Gy), while it significantly decreased after combined treatment. These findings indicated that genistein enhanced the radiosensitivity of cervical cancer HeLa cells, and the mechanisms for this action might include increase of apoptosis, decrease of survivin expression, and prolongation of cell cycle arrest.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
...染料木素在人类口服摄入后迅速被吸收。在吸收进入系统循环之前,大部分染料木素与葡萄糖醛酸结合并通过胆汁排出,进行肠肝循环...因此,染料木素的生物利用度非常有限。自由染料木素获得最大血浆浓度的时间报告为1到6小时...总共染料木素(无糖核+结合物...)为3到8小时。在其中一项研究中,使用的最低剂量(2毫克/千克体重)据称是日本日常饮食中摄入异黄酮水平的两倍多。在一项研究中,绝经妇女被给予一种含有50毫克商业异黄酮提取物的果汁、巧克力或饼干,结果显示食物基质对染料木素的吸收或尿液排泄参数没有显著影响。在一项研究中,8名妇女服用了0.4或0.8毫克/千克体重的13C标记染料木素,较高剂量的曲线下面积(AUC)不到低剂量的两倍,这表明随着剂量的增加,分数吸收率降低。
... Genistein is rapidly absorbed in humans following oral intake. Before absorption into the systemic circulation, most genistein is conjugated with glucuronic acid and excreted in the bile to undergo enterohepatic circulation ... . Therefore, genistein bioavailability is very limited. Times to obtain maximum plasma concentrations were reported at 1 to 6 hours for free genistein ... and 3 to 8 hours for total genistein (aglycone + conjugates ...). In one of the studies, the lowest dose used (2 mg/kg bw) was stated to provide more than twice the level of isoflavones ingested in a Japanese daily diet. A study in which menopausal women were given a 50 mg commercial isoflavone extract incorporated into fruit juice, chocolate, or a cookie showed no significant effect of the food matrix on genistein absorption or urinary excretion parameters. In a study in which 8 women were dosed with 0.4 or 0.8 mg/kg bw 13C-labeled genistein, the area under the curve (AUC) at the higher dose was less than double the AUC at the lower dose, suggesting a decrease in fractional absorption with increasing dose.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
摄入的染料木素和染料木苷在吸收和代谢方面存在相当大的个体差异。有一些数据表明,染料木素的生物利用度可能高于染料木苷。然而,其他数据显示,染料木苷的吸收程度对于无糖苷和葡萄糖苷形式是相似的。关于染料木素在组织分布方面的数据很少。
There is considerable individual variation in the absorption and metabolism of ingested genistin and genistein. There are some data suggesting that genistein may be more bioavailable than genistin. However, other data suggest that the extent of absorption of genistein is similar for the aglycone and the glucoside forms. There are little data available on the tissue distribution of genistein.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
最近完成的一项研究也显示了成年人在大豆挑战后对异黄酮及其代谢物的尿排泄存在个体间的差异。在这项研究中,76名志愿者被喂食了高含量(每天104+/-24毫克总异黄酮)或低含量(每天0.5+/-0.5毫克总异黄酮)的大豆饮食,持续10周。食用高含量大豆饮食的志愿者表现出大量的大豆黄酮、染料木黄酮及其代谢物的尿排泄。在高含量大豆饮食的志愿者中,34%被确定为良好的等量排泄者(每小时1000纳米摩尔)。对等量产生者和非等量产生者之间的粪便菌群进行了比较分析,然而,负责等量生产的微生物(细菌)无法被分离,因此无法被鉴定。
A recently completed study has also shown inter-individual variation in the urinary excretion of isoflavones and their metabolites following soy challenge in adults. In this study, 76 volunteers were fed either a high (104+/-24 mg total isoflavones/day) or low (0.5+/-0.5 mg total isoflavones/day) soya diet for 10 weeks. Volunteers on the high soya diet showed extensive urinary excretion of daidzein, genistein and their metabolites. Of the volunteers on the high soya diet 34% were identified as good equol excretors ( 1000 nmol/24 hours). Comparative analysis of the fecal flora between equol and non-equol producers was investigated, however, the microflora (bacteria) responsible for equol production could not be isolated and therefore, were not be identified
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
大豆异黄酮在10名健康女性体内的药代动力学是通过单次大量摄入10、20或40克大豆坚果后,血清中出现/消失的浓度曲线和尿液排泄来确定的。这些大豆坚果提供了递增的染料木黄酮(6.6、13.2和26.4毫克)和大豆苷元(9.8、19.6和39.2毫克)的共轭形式。血清中染料木黄酮和大豆苷元的峰值浓度在4-8小时后达到,消除半衰期分别为8.0小时和10.1小时。绝经前后女性在染料木黄酮和大豆苷元的药代动力学上没有差异,这表明异黄酮的吸收和处置与年龄或绝经状态无关。从血清浓度-时间曲线下的面积至无穷大(AUC(inf))和摄入的异黄酮量之间的关系来看,染料木黄酮和大豆苷元的生物利用度呈现出曲线关系。当以摄入剂量的百分比表示时,随着摄入量的增加,异黄酮在尿液中的平均排泄分数降低(分别为染料木黄酮63.2 ± 8.0、54.4 ± 8.1和44.0 ± 4.3%,大豆苷元相应地为25.2 ± 5.3、13.4 ± 2.1和15.8 ± 2.7%),这强调了非线性药代动力学的趋势。在30%的女性中识别出雌马酚作为代谢物;它一致出现在同一受试者的尿液和血液中。它的延迟出现与结肠合成一致。根据药代动力学,预期通过整天定期摄入适量的豆制品,而不是单次摄入高度浓缩的产品,可以达到最佳的稳态血清异黄酮浓度。
The pharmacokinetics of isoflavones in 10 healthy women were determined from serum appearance/disappearance concentration profiles and urinary excretions after single-bolus ingestion of 10, 20 or 40 g of soy nuts delivering increasing amounts of the conjugated forms of daidzein (6.6, 13.2 and 26.4 mg) and genistein (9.8, 19.6 and 39.2 mg). Peak serum daidzein and genistein concentrations were attained after 4-8 hr, and elimination half-lives were 8.0 and 10.1 hr, respectively. There were no differences in the pharmacokinetics of daidzein and genistein between pre- and postmenopausal women, indicating absorption and disposition of isoflavones to be independent of age or menopausal status. A curvilinear relationship was observed between the bioavailability of daidzein and genistein, apparent from the area under the curve to infinity (AUC(inf)) of the serum concentration-time profiles and the amount of isoflavones ingested. The mean fraction of the isoflavones excreted in urine decreased with increasing intake when expressed as a percentage of the administered dose (63.2 + or - 8.0, 54.4 + or - 8.1 and 44.0 + or - 4.3%, respectively, for daidzein, and correspondingly, 25.2 + or - 5.3, 13.4 + or - 2.1 and 15.8 + or - 2.7% for genistein), underscoring the trend toward nonlinear pharmacokinetics. Equol was identified as a metabolite in 30% of women; it was present consistently in urine and blood from the same subjects. Its delayed appearance was consistent with colonic synthesis. On the basis of the pharmacokinetics, optimum steady-state serum isoflavone concentrations would be expected from modest intakes of soy foods consumed regularly throughout the day rather than from a single highly enriched product.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S22,S24/25,S26
-
危险类别码:R36/38
-
WGK Germany:3
-
海关编码:2932999099
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:NR2392000
-
危险类别:IRRITANT
-
危险标志:GHS07
-
危险性描述:H302
-
储存条件:密封保存,放置于通风、干燥处,避免与氧化物接触。
SDS
模块 1. 化学品
1.1 产品标识符
: Genistein
产品名称
1.2 鉴别的其他方法
5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
4′,5,7-Trihydroxyisoflavone
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
事故响应
P301 + P312 如果吞咽并觉不适: 立即呼叫解毒中心或就医。
P330 漱口。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
别名
4′,5,7-Trihydroxyisoflavone
: C15H10O5
分子式
: 270.24 g/mol
分子量
组分 浓度或浓度范围
5,7-Dihydroxy-3-(4-hydroxyphenyl)-4-benzopyrone
<=100%
化学文摘登记号(CAS 446-72-0
No.) 207-174-9
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
避光。 贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
建议的贮存温度: -20 °C
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 淡黄
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
> 280 °C
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
发光。
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 小鼠 - 500 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
细胞突变性-体外试验 - 人 - 淋巴细胞
微核测试
细胞突变性-体外试验 - 人 - 其他细胞类型
DNA损伤
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: NR2392000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 16. 其他信息
进一步信息
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
制备方法与用途
性状
染料木素为浅黄色结晶性粉末,气味轻微,味道苦涩。
用途染料木素具有雌激素和抗雌激素性质,同时具备抗氧化作用。它能够抑制酪氨酸蛋白激酶(PTK)的活性及拓扑异构酶Ⅱ的活性,并能诱发细胞程序性死亡、提高抗癌药物效果以及抑制血管生成等。作为一种有潜力的癌症化学预防剂,染料木素在抗肿瘤和治疗相关疾病方面展现出广泛的应用前景。
理化性质淡黄色树枝状针晶粉末。熔点为297℃-298℃;溶解于常用的有机溶剂中几乎不溶于水,但在稀碱溶液中会呈现黄色。
药理作用染料木素(也称为金雀异黄酮)是一种多酚类化合物,在大豆和红三叶草等植物中发现。其分子结构类似17β-雌二醇,并具有高亲和性与雌激素受体结合的特性,从而抑制酪氨酸蛋白激酶(PTK)和拓扑异构酶Ⅱ的活性。染料木素不仅能诱发细胞程序性死亡、增强抗癌药效、抑制血管生成等作用,还是一种很有潜力的癌症化学预防剂。
金雀异黄酮对人体健康的好处已经得到广泛研究,并且具有显著影响。作为一种植物雌激素,它不仅对抗氧化有帮助,还能调节体内多种疾病状态,如更年期综合征、骨质疏松和血脂升高等问题。
化学性质白色固体。
用途- 抗肿瘤
- 抗真菌
- 降血脂
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 鹰嘴豆芽素A 5,7-Dihydroxy-3-(4-methoxy-phenyl)-chromen-4-on 491-80-5 C16H12O5 284.268 —— 5-hydroxy-7-(methoxymethoxy)-3-(4-methoxyphenyl)-4H-chromen-4-one 1005418-09-6 C18H16O6 328.321 —— [5-hydroxy-3-(4-hydroxyphenyl)-4-oxochromen-7-yl]dihydrogen phosphate —— C15H11O8P 350.221 —— 3-(4-hydroxyphenyl)-5,7-bis(methoxymethoxy)-4H-chromen-4-one 1236208-91-5 C19H18O7 358.348 —— 5,7-dihydroxy-3-(4-hydroxy-phenyl)-4-oxo-4H-chromene-2-carboxylic acid 22151-32-2 C16H10O7 314.251 槐角苷 sophoricoside 152-95-4 C21H20O10 432.384 染料木苷 genistin 529-59-9 C21H20O10 432.384 5,7-二羟基色原酮 5,7-dihydroxy-chromen-4-one 31721-94-5 C9H6O4 178.144 —— sphaerobioside —— C27H30O14 578.527 6-O-乙酰染料木苷 genistein-7-O-β-D-(6''-O-acetylglucopyranoside) 73566-30-0 C23H22O11 474.421 —— genistein 5-glucoside 128508-06-5 C21H20O10 432.384 丙二酰染料木苷 6''-O-malonylgenistin 51011-05-3 C24H22O13 518.431 —— 7,4'-di-O-<4-O-β-D-glucopyranosyl-β-D-apiofuranoside> genisteol 78693-95-5 C37H46O23 858.758 豆苷 daidzin 552-66-9 C21H20O9 416.384 —— chevalierinoside C 1673575-50-2 C26H28O14 564.5 —— genistein 7-O-(6-O-((E)-caffeoyl)-β-D-glucopyranoside) 1401251-26-0 C30H26O13 594.529 —— genistein 7-O-(2"-p-coumaroyl-β-D-glucopyranoside) —— C30H26O12 578.529 —— 3-iodo-5,7-bis(methoxymethoxy)-4H-chromen-4-one 1236208-90-4 C13H13IO6 392.147 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 鹰嘴豆芽素A 5,7-Dihydroxy-3-(4-methoxy-phenyl)-chromen-4-on 491-80-5 C16H12O5 284.268 樱黄素 prunetin 552-59-0 C16H12O5 284.268 3',4',5,7-四羟基异黄酮 orobol 480-23-9 C15H10O6 286.241 4’,7-二甲氧基-5-羟基异黄酮 4',7-dimethoxy-5-hydroxyisoflavone 34086-51-6 C17H14O5 298.295 —— 4'-O-carboxymethyl-genistein —— C17H12O7 328.278 —— genistein 4'-O-phosphate —— C15H11O8P 350.221 —— 6-Hydroxygenistein 13539-26-9 C15H10O6 286.241 —— 4'-(dimethylallyl)genistein —— C20H18O5 338.36 —— 5,7-Bis[(~2~H)hydroxy]-3-[4-(~2~H)hydroxyphenyl]-4H-1-benzopyran-4-one 136466-46-1 C15H10O5 273.218 —— 7-O-difluoromethylgenistein 848565-56-0 C16H10F2O5 320.249 —— 7-(tert-butoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1610737-64-8 C19H18O5 326.349 —— 7-(2-bromoethoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 862255-00-3 C17H13BrO5 377.191 5,7-二甲氧基-3-(4-甲氧基苯基)-4H-1-苯并吡喃-4-酮 4',5,7-trimethoxyisoflavone 1162-82-9 C18H16O5 312.322 —— 7,4'-gem-difluoromethylgenistein 848565-57-1 C17H10F4O5 370.257 —— 7-(3-bromopropoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 862255-02-5 C18H15BrO5 391.218 —— 4',7-diallyloxy-5-hydroxygenistein —— C21H18O5 350.371 —— 7-(4-bromobutoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1048693-51-1 C19H17BrO5 405.245 —— 7-carboxymethoxy-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one —— C17H12O7 328.278 3'-O-甲基香豌豆苷元 3'-O-methylorobol 36190-95-1 C16H12O6 300.268 —— [5-hydroxy-3-(4-hydroxyphenyl)-4-oxochromen-7-yl]dihydrogen phosphate —— C15H11O8P 350.221 —— 7-acetoxy-4',5-dihydroxygenistein —— C17H12O6 312.279 4’-O-叔-丁基二甲基硅烷基染料木碱 3-[4-(tert-butyldimethylsilyloxy)phenyl]-5,7-dihydroxy-4H-chromen-4-one 470666-97-8 C21H24O5Si 384.504 —— 7,4'-di-O-prenylgenistein 51225-25-3 C25H26O5 406.478 —— 4',7-dioctyloxy-5-hydroxygenistein —— C31H42O5 494.671 —— 4',7-didecyloxy-5-hydroxygenistein —— C35H50O5 550.779 3'-O-甲基红车轴草素 3-(3,4-dimethoxyphenyl)-5,7-dihydroxy-4H-chromen-4-one 53084-11-0 C17H14O6 314.295 7,4'-二乙酰氧基-5-羟基异黄酮 4-(7-acetoxy-5-hydroxy-4-oxo-4H-chromen-3-yl)phenyl acetate 65388-04-7 C19H14O7 354.316 —— 4'-O-hexanoylgenistein —— C21H20O6 368.386 8-羟基染料木黄酮 8-Hydroxygenistein 13539-27-0 C15H10O6 286.241 —— 4'-(1-methyl-1-carboxylethoxy)-5,7-dihydroxyisoflavone 1204747-80-7 C19H16O7 356.332 —— 5,7-diethoxy-3-(4-ethoxyphenyl)chromen-4-one 187656-35-5 C21H22O5 354.403 —— 7-difluoromethoxy-5,4′-dimethoxygenistein 848565-58-2 C18H14F2O5 348.303 —— genistein-4'-monostearate —— C33H44O6 536.709 —— 5-hydroxy-3-(4-hydroxyphenyl)-7-(4-(pyrrolidin-1-yl)butoxy)-4H-chromen-4-one 1048693-67-9 C23H25NO5 395.455 —— 5-hydroxy-3-(4-hydroxyphenyl)-7-(3-(pyrrolidin-1-yl)propoxy)-4H-chromen-4-one 947603-49-8 C22H23NO5 381.428 —— 5-hydroxy-3-(4-hydroxyphenyl)-7-(2-(pyrrolidin-1-yl)ethoxy)-4H-chromen-4-one 947603-39-6 C21H21NO5 367.401 —— 4',5,7-tri-O-trimethylsilyloxyisoflavone 148356-36-9 C24H34O5Si3 486.787 —— 7-O-geranyl genistein 1072940-14-7 C25H26O5 406.478 —— 7-O-carbo-tert-butoxymethylgenistein —— C21H20O7 384.386 —— genistein-4'-monooleate —— C33H42O6 534.693 —— 7-(tert-butyldimethylsilyloxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one —— C21H24O5Si 384.504 —— 5,7,8,3',4'-pentahydroxy-8-methylisoflavone —— C15H10O7 302.24 —— 7-((4-fluorobenzyl)oxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1093389-85-5 C22H15FO5 378.357 —— 7-(tert-butyldimethylsilyloxy)-3-[4-(tert-butyldimethylsilyloxy)phenyl]-5-hydroxy-4H-chromen-4-one 470666-98-9 C27H38O5Si2 498.767 —— 3-(4-(3-(benzyl(methyl)amino)propoxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-19-6 C26H25NO5 431.488 —— 4',7-dipivaloyloxy-5-hydroxyisoflavone 444643-69-0 C25H26O7 438.477 —— 7-difluoro-5,4'-diethoxygenistein 848565-59-3 C20H18F2O5 376.357 —— 4'-(1-methyl-1-(ethoxycarbonyl)ethoxy)-5,7-dihydroxyisoflavone 1204747-79-4 C21H20O7 384.386 —— genistein-7-monostearate —— C33H44O6 536.709 —— 4-(5-acetoxy-7-hydroxy-4-oxo-4H-chromen-3-yl)phenyl acetate 23050-36-4 C19H14O7 354.316 —— 7-(2-(1H-imidazol-1-yl)ethoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 947603-43-2 C20H16N2O5 364.357 —— Tert-butyl [4-[5-hydroxy-7-[(2-methylpropan-2-yl)oxycarbonyloxy]-4-oxochromen-3-yl]phenyl] carbonate 1038994-86-3 C25H26O9 470.476 —— 3-(4-(3-(benzyl(ethyl)amino)propoxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-25-4 C27H27NO5 445.515 —— genistein-4',7-distearate —— C51H78O7 803.176 —— 3-(4-(4-(benzyl(methyl)amino)butoxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-44-7 C27H27NO5 445.515 —— 7-(3-(1H-imidazol-1-yl)propoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 947603-53-4 C21H18N2O5 378.384 —— 4',5,7-Triacetoxyisoflavone 5995-97-1 C21H16O8 396.353 —— 8-chlorogenistein 145400-84-6 C15H9ClO5 304.686 —— 4',5,7-tri-O-benzylgenistein —— C36H28O5 540.615 —— 3-(4-((6-(benzyl(methyl)amino)hexyl)oxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-72-1 C29H31NO5 473.569 —— genistein-7-monooleate —— C33H42O6 534.693 —— 7-(3-(benzyl(methyl)amino)propoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1439923-38-2 C26H25NO5 431.488 —— 3-(4-(4-(benzyl(ethyl)amino)butoxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-52-7 C28H29NO5 459.542 —— 7-(4-(1H-imidazol-1-yl)butoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1048693-82-8 C22H20N2O5 392.411 —— genistein-4',7-dioleate —— C51H74O7 799.144 —— 3-(4-((6-(benzyl(ethyl)amino)hexyl)oxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-78-7 C30H33NO5 487.596 —— 7-difluoromethyl-5,4'-diheptyloxygenistein 848565-62-8 C30H38F2O5 516.626 —— 7-difluoromethyl-5,4'-didecyloxygenistein 848565-64-0 C36H50F2O5 600.787 —— 7-difluoromethyl-5,4'-di-n-octyloxygenistein 848565-63-9 C32H42F2O5 544.679 —— 4-(7-acetyloxy-5-((3-methylbut-2-en-l-yl))-4-oxo-4H-chromen-3-yl)phenyl acetate 583037-95-0 C24H22O7 422.434 —— 7-(3-(benzyl(ethyl)amino)propoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1439912-98-7 C27H27NO5 445.515 —— 7-(4-(benzyl(methyl)amino)butoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1439913-13-9 C27H27NO5 445.515 —— 3'-nitrogenistein —— C15H9NO7 315.239 —— 7-((6-(benzyl(methyl)amino)hexyl)oxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1439913-34-4 C29H31NO5 473.569 —— 7-(4-(benzyl(ethyl)amino)butoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1439913-20-8 C28H29NO5 459.542 Wighteone; 5,7,4'三羟基-6-异戊烯基异黄酮 erythrinin B 51225-30-0 C20H18O5 338.36 —— 5,4'-di-O-hexanoyl-genistein 918158-58-4 C27H30O7 466.531 —— 7-difluoromethyl-5,4'-dibenzyloxygenistein 848565-61-7 C30H22F2O5 500.498 —— 7-(4-(benzyl(ethyl)amino)butoxy)-3-(4-(4-(benzyl(ethyl)amino)butoxy)phenyl)-5-hydroxy-4H-chromen-4-one 1439915-83-9 C41H48N2O5 648.843 —— 7-((6-(benzyl(ethyl)amino)hexyl)oxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1439913-40-2 C30H33NO5 487.596 黄豆黄素 glycitein 40957-83-3 C16H12O5 284.268 —— 5,4',7-tri-O-hexanoylgenistein 918158-57-3 C33H40O8 564.676 —— 3-(4-hydroxyphenyl)-7-benzenesulfonyloxy-5-hydroxy-4H-chromen-4-one 1041727-51-8 C21H14O7S 410.404 —— 5,7-dihydroxy-3-(4-(3-((2-methoxybenzyl)(methyl)amino)propoxy)phenyl)-4H-chromen-4-one 1439910-20-9 C27H27NO6 461.514 —— myrsininone A —— C25H26O5 406.478 —— 3-(4-benzenesulfonyloxyphenyl)-7-benzenesulfonyloxy-5-hydroxy-4H-chromen-4-one 1041727-54-1 C27H18O9S2 550.567 —— 5,7,3',4'-tetrahydroxy-8-methylisoflavone —— C16H12O6 300.268 —— 3-(4-(3-(ethyl(2-methoxybenzyl)amino)propoxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-26-5 C28H29NO6 475.541 —— 5,7-dihydroxy-3-(4-(4-((2-methoxybenzyl)(methyl)amino)butoxy)phenyl)-4H-chromen-4-one 1439910-45-8 C28H29NO6 475.541 金雀异黄酮-D4 5,7-dihydroxy-3-(4-hydroxyphenyl-2,3,5,6-d4)-4H-1-benzopyran-4-one 187960-08-3 C15H10O5 274.21 —— 6,8-dichlorogenistein 145400-86-8 C15H8Cl2O5 339.132 —— 5,7-dihydroxy-3-(4-((6-((2-methoxybenzyl)(methyl)amino)hexyl)oxy)phenyl)-4H-chromen-4-one 1439910-73-2 C30H33NO6 503.595 —— 3-(4-(4-(ethyl(2-methoxybenzyl)amino)butoxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-53-8 C29H31NO6 489.568 —— 5-hydroxy-3-(4-hydroxyphenyl)-7-(3-((2-methoxybenzyl)(methyl)amino)propoxy)-4H-chromen-4-one 1439912-93-2 C27H27NO6 461.514 —— 5-hydroxy-3-(4-hydroxyphenyl)-7-morpholino-4H-chromen-4-one 1610737-75-1 C19H17NO5 339.348 —— 3-(4-((6-(ethyl(2-methoxybenzyl)amino)hexyl)oxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one 1439910-79-8 C31H35NO6 517.622 大豆异黄酮 isoflavone 574-12-9 C15H10O2 222.243 —— 5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl-4-methylbenzenesulfonate 251985-66-7 C22H16O7S 424.431 —— 7-(3-(ethyl(2-methoxybenzyl)amino)propoxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one 1439912-99-8 C28H29NO6 475.541 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:Synthesis and Biological Activity of Isoflavone Derivatives from Chickpea as Potent Anti-Diabetic Agents摘要:从鹰嘴豆中合成了一系列新型异黄酮衍生物。通过质子核磁共振(1H-NMR)、碳-13(13C)-NMR 和质谱(MS)光谱分析确定了这些衍生物的结构。利用胰岛素抗性(IR)HepG2 细胞模型对这些化合物的抗糖尿病活性进行了评估。此外,还简要讨论了这些衍生物的结构-活性关系。化合物 1c、2h、3b 和 5 以及染料木素在 IR-HepG2 细胞中表现出显著的葡萄糖消耗增强效应。此外,与单个化合物相比,染料木素、2h 和 3b 的组合(组合 6)以及 3b、染料木素和 1c 的组合(组合 10)具有更好的抗糖尿病活性。在相同剂量下,组合 10 与阳性对照的效果没有差异(P > 0.05)。此外,我们还发现组合 10 和组合 6 在高浓度葡萄糖条件下对 HUVEC(人脐静脉内皮细胞)的保护作用存在差异。组合 10 的保护作用强于组合 6,这表明组合 10 在未来的研究中可能具有更好的降糖活性。这项研究为进一步设计和发现抗糖尿病药物提供了有用的线索。DOI:10.3390/molecules200917016
-
作为产物:描述:参考文献:名称:两种生物活性异黄酮糖苷Talosin A和B的合成摘要:Talosin A和B,即染料木素7‐ O ‐ α ‐ L ‐6‐脱氧塔洛吡喃糖苷和染料木素4′,7‐di‐ O ‐ α ‐ L ‐‐6‐脱氧塔洛吡喃糖苷,显示出优异的抗真菌和抗炎活性,简明扼要。DOI:10.1002/cjoc.201090291
-
作为试剂:描述:参考文献:名称:(Bio)-synthesis of Au NPs from soy isoflavone extracts as a novel assessment tool of their antioxidant capacity摘要:number(n=1)或多个(n=2)金纳米颗粒。DOI:10.1039/c3ra45180a
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] COMPOUNDS AS MODULATORS OF TIGIT SIGNALLING PATHWAY<br/>[FR] COMPOSÉS MODULATEURS DE LA VOIE DE SIGNALISATION DE TIGIT申请人:AURIGENE DISCOVERY TECH LTD公开号:WO2018047139A1公开(公告)日:2018-03-15The present invention relates to compound of formula (I) as therapeutic agents to modulate the TIGIT signalling pathway. The invention also encompasses the use of the compound of formula (I) or a stereoisomer thereof or a pharmaceutically acceptable salt thereof for the treatment of diseases or disorders mediated by TIGIT.本发明涉及一种化合物,其化学式为(I),作为调节TIGIT信号通路的治疗剂。该发明还涵盖了利用化合物(I)或其立体异构体或其药学上可接受的盐来治疗由TIGIT介导的疾病或紊乱。
-
Substituted 1-benzoyl-3-cyano-pyrrolo [1,2-a] quinolines and analogs as activators of caspases and inducers of apoptosis申请人:Cai Xiong Sui公开号:US20050014759A1公开(公告)日:2005-01-20The present invention is directed to substituted 1-benzoyl-3-cyano-pyrrolo[1,2-a]quinolines and analogs thereof, represented by the general Formula I: wherein R 1 —R 8 , L, Q, dash line and Ar are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. Therefore, the activators of caspases and inducers of apoptosis of this invention can be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.本发明涉及取代的1-苯甲酰基-3-氰基-吡咯并[1,2-a]喹啉及其类似物,由通用式I表示: 其中R1-R8,L,Q,虚线和Ar在此定义。本发明还涉及发现具有式I的化合物是caspase的激活剂和凋亡诱导剂。因此,本发明的caspase激活剂和凋亡诱导剂可用于诱导在各种临床病况中发生未受控制的异常细胞生长和扩散的细胞死亡。
-
[EN] alpha7 NICOTINIC ACETYLCHOLINE RECEPTOR MODULATORS AND USES THEREOF-I<br/>[FR] MODULATEURS DU RÉCEPTEUR Alpha7 NICOTINIQUE D'ACÉTYLCHOLINE ET LEURS UTILISATIONS申请人:BIONOMICS LTD公开号:WO2014019023A1公开(公告)日:2014-02-06The present invention relates to chemical compounds of formula (I), with the substituents as described in the specification, useful in the positive modulation of the alpha 7 nicotinic acetylcholine receptor (α7 nAChR). The invention also relates to the use of these compounds in the treatment or prevention of a broad range of diseases in which the positive modulation of α7 nAChR is advantageous, including neurodegenerative and neuropsychiatric diseases and also neuropathic pain and inflammatory diseases.
-
SYNTHETIC SUBSTRATES FOR ENZYMES THAT CATALYZE REACTIONS OF MODIFIED CYSTEINES AND RELATED METHODS申请人:The University of Chicago公开号:US20180147250A1公开(公告)日:2018-05-31Synthetic probes for detecting the activity of enzymes that catalyze reactions of post-translationally modified cysteine residues are described. The probes include “turn-on” probes that include a carbamate linkage that is cleaved via an intramolecular reaction with a free thiol produced by an enzyme catalyzed activity. The probes also include ratiometric, Michael addition-based probes that respond to enzymatic activity by a change in structure that results in a change in fluorescence properties. Methods of using the probes to detect enzymatic activity and disease are described. For example, the probes can be used to detect enzymatic activity in a variety of samples, including live cells and heterogeneous tissues. In addition, prodrugs that can be activated by enzymes that catalyze reactions of post-translationally modified cysteine residues and methods of using the prodrugs to treat disease are described.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄豆黄苷
黄豆黄素
黄豆苷元-D6
黄豆苷元-4,7-二葡糖苷
黄芪异黄烷苷,7,2'-二羟基-3',4'-二甲氧基异黄烷
黄羽扇豆魏特酮
黄细心酮 E
黄细心酮 B
鹰嘴豆芽素A
鸢尾黄酮甲素
鸢尾黄酮乙素
鸢尾黄素
鸢尾黄素
鸢尾苷
鸡豆黄素配糖物
鱼藤醇酮
鱼藤酮
鱼藤二酮
魚藤素
高紫檀素; 3,9-二甲氧基紫檀碱
高丽槐素乙酸酯
高丽槐素
顺式奥美昔芬
雌马酚
雌马酚
降香黄烃
阿比西尼亚桐素II;(6aR,11aR)-6a,11a-二氢-2,10-双(3-甲基-2-丁烯-1-基)-6H-苯并呋喃并[3,2-c][1]苯并吡喃-3,9-二醇
金雀异黄酮-D4
金雀异黄素4'-β-D-葡糖醛酸
野鹫尾苷
野鸢尾黄素
豌豆素
豆苷
西卡宁
西北甘草异黄酮
补骨脂异黄酮
补骨脂定
蟛蜞菊内酯
葛花苷
葛花宁
葛根素芹菜苷
葛根素-4'-Β-D-葡萄糖苷
葛根素
菜豆蛋白
菜豆素
菜豆异黄烷
菜豆双氢异黄酮
荧光增白剂 236
茚并[2,1-b]色烯
苯并[b]茚并[1,2-e]吡喃-6-甲醛