甘露醇 | 87-78-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:167-170 °C(lit.)
-
比旋光度:141 º (c= USP-directives)
-
沸点:295°C
-
密度:1.52
-
闪点:290-295°C/3.5mm
-
溶解度:H2O:1 Mat 20 °C,透明,无色
-
最大波长(λmax):λ: 260 nm Amax: 0.04λ: 280 nm Amax: 0.04
-
LogP:-3.262 (est)
-
物理描述:D-mannitol appears as odorless white crystalline powder or free-flowing granules. Sweet taste. (NTP, 1992)
-
颜色/状态:Orthorhombic needles from alc
-
气味:Odorless
-
味道:Sweetish taste
-
稳定性/保质期:
Mannitol 25% (Invenex) was chemically and physically stable after five autoclavings at 250 °F for 15 min.
-
旋光度:Specific optical rotation: -0.49 deg @ 25 °C/D (water)
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
折光率:Index of refraction: 1.3330
-
Caco2细胞的药物渗透性:-6.21
-
解离常数:pKa = 13.50 @ 18 °C
-
碰撞截面:141 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):-3.1
-
重原子数:12
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:121
-
氢给体数:6
-
氢受体数:6
ADMET
安全信息
-
TSCA:Yes
-
海关编码:2905430000
-
安全说明:S22,S24/25
-
WGK Germany:3
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
制备方法与用途
D-甘露醇(D-Mannitol),又称为D-甘露糖醇,是一种六元醇。广泛存在于植物或植物分泌物中,可通过从海带或海藻提取获得;也可以通过催化加氢的方法制得葡萄糖或蔗糖。
来源天然品在多种植物中存在,如海藻、柿饼表面的白粉、食用菌类、地衣类、洋葱及胡萝卜等。木犀科植物花白蜡树(Fraxinus ornus)的树液中也含有甘露聚糖,其中甘露糖醇含量为30%~50%。
应用D-甘露醇可用作低热量甜味剂;胶姆糖及糖果的防粘剂;营养增补剂及组织改良剂;保湿剂。
毒性与使用限量ADI无特殊规定(FAO/WHO,2001)。LD₅₀为17.3g/kg(大鼠,经口)。
-
EEC:不得作为婴幼儿特殊强化食品。可用于糖果、冰淇淋和胶姆糖。
-
FDA(21CFR§180.25):
- 薄荷糖98%
- 硬糖5%
- 止咳糖5%
- 胶姆糖31%
- 软糖40%
- 蜜饯和冰霜8%
- 未标准化果酱和果冻15%
- 其他食品2.5%
-
日本:规定只准用于胶姆糖和饴糖类制品的防粘。
-
GB/T 2760 2000:无糖口香糖,200g/kg。
D-甘露醇为无色至白色针状或斜方柱状晶体或结晶性粉末。无臭,具有清凉甜味,甜度约为蔗糖的57%~72%,每克产生8.37J热量(约为葡萄糖的一半),含少量山梨糖醇。相对密度1.49,旋光率[α]₂₀ -0.40°(10%水溶液),吸湿性极小,水溶液稳定。对稀酸、稀碱稳定,不被空气中氧氧化。溶于水(5.6g/100ml,20℃)及甘油(5.5g/100mL)。略溶于乙醇(1.2g/100mL),溶于热乙醇,几乎不溶于大多数其他常用有机溶剂。20%水溶液的pH值为5.5~6.5。
用途D-甘露糖醇在医药上是良好的利尿剂、降低颅内压及眼内压药物,并用于治疗肾药、脱水剂、食糖代用品,也用作片剂赋形剂和固体、液体的稀释剂。甘露醇注射液作为高渗透降压药,在临床上抢救特别是脑部疾患时常用。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 山梨醇 D-sorbitol 50-70-4 C6H14O6 182.174 2,4:3,5-二-O-苄亚基-L-艾杜糖醇 sorbitol 45007-61-2 C6H14O6 182.174 —— Xylitol 87-99-0 C5H12O5 152.147 —— D-Arabitol 488-82-4 C5H12O5 152.147 D-甘露糖 D-Mannose 3458-28-4 C6H12O6 180.158 葡萄糖 D-glucose 50-99-7 C6H12O6 180.158 口服葡萄糖 D-Galactose 59-23-4 C6H12O6 180.158 L-阿卓糖 L-altrose 1949-88-8 C6H12O6 180.158 D-艾杜糖 D-idose 5978-95-0 C6H12O6 180.158 α-D-(+)-塔罗糖 D-talose 2595-98-4 C6H12O6 180.158 L-(-)-艾杜糖 idose 5934-56-5 C6H12O6 180.158 古洛糖 L-gulose 6027-89-0 C6H12O6 180.158 —— D-alpha-glucoheptose 94667-85-3 C7H14O7 210.184 L-阿拉伯糖 L-arabinose 5328-37-0 C5H10O5 150.131 D-木糖 D-xylose 58-86-6 C5H10O5 150.131 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 L-甘露醇 L-mannitol 643-01-6 C6H14O6 182.174 山梨醇 D-sorbitol 50-70-4 C6H14O6 182.174 —— D-galactitol 608-66-2 C6H14O6 182.174 —— allitol 488-44-8 C6H14O6 182.174 D-艾杜糖醇 D-iditol 25878-23-3 C6H14O6 182.174 —— allo-inositol 643-10-7 C6H12O6 180.158 —— 1,2,3,5,6-hexanepentaol 93132-95-7 C6H14O5 166.174 —— Xylitol 87-99-0 C5H12O5 152.147 —— 1,4,5-hexanetriol 140946-33-4 C6H14O3 134.175 D-甘露糖 D-Mannose 3458-28-4 C6H12O6 180.158 L-阿卓糖 L-altrose 1949-88-8 C6H12O6 180.158 葡萄糖 D-glucose 50-99-7 C6H12O6 180.158 口服葡萄糖 D-Galactose 59-23-4 C6H12O6 180.158 D-阿洛糖 D-Allose 6038-51-3 C6H12O6 180.158 L-(-)-艾杜糖 idose 5934-56-5 C6H12O6 180.158 1,2,5,6-四羟基己烷 1,2,5,6-hexanetetrol 5581-21-5 C6H14O4 150.175 二脱水甘露醇 1,2:5,6-dianhydro-D-mannitol 19895-66-0 C6H10O4 146.143 —— dimannitol —— C12H26O11 346.332 己烷-1,2,5-三醇 1,2,5-hexanetriol 10299-30-6 C6H14O3 134.175 —— 1,6-anhydro-D-mannitol 908000-43-1 C6H12O5 164.158 L-鼠李糖 L-Rhamnose 3615-41-6 C6H12O5 164.158 —— 1,6-diamino-1,6-dideoxy-D-mannitol 41111-65-3 C6H16N2O4 180.204 二溴甘露醇 mitobronitol 488-41-5 C6H12Br2O4 307.967 —— dibromodulcitol 10318-26-0 C6H12Br2O4 307.967 —— 1,6-dichloro-1,6-dideoxy-D-mannitol 7251-85-6 C6H12Cl2O4 219.065 —— 1,6-dichloro-1,6-dideoxy-mannitol 196812-16-5 C6H12Cl2O4 219.065 1,2,6-己三醇 hexane-1,2,6-triol 106-69-4 C6H14O3 134.175 —— (2R,5R)-2,5-dimethylmannitol 170889-88-0 C8H18O6 210.227 阿拉伯糖 D-Arabinose 147-81-9 C5H10O5 150.131 —— Hexa-O-methyl-D-mannit 20746-37-6 C12H26O6 266.335 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:哒嗪酮取代的1,3,4-噻二唑,-1,3,4-恶二唑和-1,2,4-三唑的合成摘要:制备了几种1-(1-芳基-1,4-二氢-3-羧基-6-甲基哒嗪-4-酮)-4-芳基硫代氨基脲及其相应的恶二唑,噻二唑和三唑衍生物,并通过光谱数据对其进行了表征。 。初步的生物学测试表明,一些新化合物具有良好的抗真菌活性。DOI:10.1002/jhet.5570380431
-
作为产物:参考文献:名称:水解合成糖醇 氢化 负载金属催化剂上纤维素的制备摘要:纤维素转化为 山梨糖醇 和相关的糖化合物超过 水耐久的碳载铂 催化剂 在水之下 氢化条件。用球预处理纤维素铣削 有效降低纤维素的结晶度和粒径,从而导致纤维素高转化为 山梨糖醇 和 甘露醇。的选择性山梨糖醇 通过使用不含氯的金属前体来增加 催化剂 制备为残留Cl的 催化剂促进副反应。纤维素向山梨糖醇包括通过水溶性纤维素水解为葡萄糖寡糖 然后连续氢化 葡萄糖 到 山梨糖醇。这水解 纤维素是决定速率的步骤,而铂 催化剂 促进水解和氢化步骤。DOI:10.1039/c0gc00666a
-
作为试剂:参考文献:名称:Selective Synthesis of Mono-substituted Ureas in Low Melting Citric Acid-Urea-Mannitol Mixture摘要:DOI:10.1080/00304948.2014.944408
文献信息
-
[EN] BENZAMIDE OR BENZAMINE COMPOUNDS USEFUL AS ANTICANCER AGENTS FOR THE TREATMENT OF HUMAN CANCERS<br/>[FR] COMPOSÉS BENZAMIDE OU BENZAMINE À UTILISER EN TANT QU'ANTICANCÉREUX POUR LE TRAITEMENT DE CANCERS HUMAINS申请人:UNIV TEXAS公开号:WO2017007634A1公开(公告)日:2017-01-12The described invention provides small molecule anti-cancer compounds for treating tumors that respond to cholesterol biosynthesis inhibition. The compounds selectively inhibit the cholesterol biosynthetic pathway in tumor-derived cancer cells, but do not affect normally dividing cells.
-
[EN] TARGETED DELIVERY AND PRODRUG DESIGNS FOR PLATINUM-ACRIDINE ANTI-CANCER COMPOUNDS AND METHODS THEREOF<br/>[FR] ADMINISTRATION CIBLÉE ET CONCEPTIONS DE PROMÉDICAMENTS POUR COMPOSÉS ANTICANCÉREUX À BASE DE PLATINE ET D'ACRIDINE ET MÉTHODES ASSOCIÉES申请人:WAKE FOREST SCHOOL OF MEDICINE公开号:WO2013033430A1公开(公告)日:2013-03-07Acridine containing cispiaiin compounds have been disclosed that show greater efficacy against cancer than other cisplatin compounds. Methods of delivery of those more effective eisp!aiin compounds to the nucleus in cancer ceils is disclosed using one or more amino acids, one or more sugars, one or more polymeric ethers, C i^aikylene-phenyl-NH-C(0)-R.15, folic acid, av03 iniegriii RGD binding peptide, tamoxifen, endoxifen, epidermal growth factor receptor, antibody conjugates, kinase inhibitors, diazoles, triazol.es, oxazoies, erlotinib, and/or mixtures thereof; wherein R]§ is a peptide.
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] AZA PYRIDONE ANALOGS USEFUL AS MELANIN CONCENTRATING HORMONE RECEPTOR-1 ANTAGONISTS<br/>[FR] ANALOGUES D'AZAPYRIDONE UTILES COMME ANTAGONISTES DU RÉCEPTEUR 1 DE L'HORMONE CONCENTRANT LA MÉLANINE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2010104818A1公开(公告)日:2010-09-16MCHR1 antagonists are provided having the following Formula (I): A1 and A2 are independently C or N; E is C or N; Q1, Q2, and Q3 are independently C or N provided that at least one of Q1, Q2, and Q3 is N but not more than one of Q1, Q2, and Q3 is N; D1 is a bond, -CR8R9 X-, -XCR8R9-, -CHR8CHR9-, -CR10=CR10'-, -C≡C-, or 1,2-cyclopropyl; X is O, S or NR11; R1, R2, and R3 are independently selected from the group consisting of hydrogen, halogen, lower alkyl, lower cycloalkyl, -CF3, -OCF3, -OR12 and -SR12; G is O, S or -NR15; D2 is lower alkyl, lower cycloalkyl, lower alkylcycloalkyl, lower cycloalkylalkyl, lower cycloalkoxyalkyl or lower alkylcycloalkoxy or when G is NR15, G and D2 together may optionally form an azetidine, pyrrolidine or piperidine ring; Z1 and Z2 are independently hydrogen, lower alkyl, lower cycloalkyl, lower alkoxy, lower cycloalkoxy, halo, -CF3, -OCONR14R14', -CN, -CONR14R14', -SOR12, -SO2R12, -NR14COR14', -NR14CO2R14', -CO2R12, NR14SO2R12 or COR12; R5, R6, and R7 are independently selected from the group consisting of hydrogen lower alkyl, lower cycloalkyl, -CF3, -SR12, lower alkoxy, lower cycloalkoxy, -CN, -CONR14R14', SOR12, SO2R12, NR14COR14', NR14CO2R12, CO2R12, NR14SO2R12 and -COR12; R8, R9, R10, R10', R11 are independently hydrogen or lower alkyl; R12 is lower alkyl or lower cycloalkyl; R14 and R14' are independently H, lower alkyl, lower cycloalkyl or R14 and R14' together with the N to which they are attached form a ring having 4 to 7 atoms; and R15 is independently selected from the group consisting of hydrogen and lower alkyl. Such compounds are useful for the treatment of MCHR1 mediated diseases, such as obesity, diabetes, IBD, depression, and anxiety.MCHR1拮抗剂具有以下化学式(I):A1和A2独立地为C或N;E为C或N;Q1、Q2和Q3独立地为C或N,但至少其中一个为N,但不超过一个为N;D1为键,-CR8R9 X-,-XCR8R9-,-CHR8CHR9-,-CR10=CR10'-,-C≡C-,或1,2-环丙基;X为O、S或NR11;R1、R2和R3独立地从氢、卤素、低烷基、低环烷基、-CF3、-O 、-OR12和-SR12组成的群体中选择;G为O、S或-NR15;D2为低烷基、低环烷基、低烷基环烷基、低环烷基烷基、低环烷氧基烷基或低烷基环烷氧基,或当G为NR15时,G和D2一起可以选择形成氮杂环丙烷、吡咯烷或哌啶环;Z1和Z2独立地为氢、低烷基、低环烷基、低烷氧基、低环烷氧基、卤素、- 、-OCONR14R14'、-CN、-CONR14R14'、-SOR12、-SO2R12、-NR14COR14'、-NR14CO2R14'、-CO2R12、NR14SO2R12或COR12;R5、R6和R7独立地从氢、低烷基、低环烷基、- 、-SR12、低烷氧基、低环烷氧基、-CN、-CONR14R14'、SOR12、SO2R12、NR14COR14'、NR14CO2R12、CO2R12、NR14SO2R12和-COR12组成的群体中选择;R8、R9、R10、R10'、R11独立地为氢或低烷基;R12为低烷基或低环烷基;R14和R14'独立地为H、低烷基、低环烷基或R14和R14'与其连接的N一起形成具有4至7个原子的环;R15独立地从氢和低烷基组成的群体中选择。这些化合物对于治疗MCHR1介导的疾病,如肥胖症、糖尿病、炎症性肠病、抑郁症和焦虑症非常有用。
-
DISUBSTITUTED TRIFLUOROMETHYL PYRIMIDINONES AND THEIR USE申请人:BAYER PHARMA AKTIENGESELLSCHAFT公开号:US20160221965A1公开(公告)日:2016-08-04The present application relates to novel 2,5-disubstituted 6-(trifluoromethyl)pyrimidin-4(3H)-one derivatives, to processes for their preparation, to their use alone or in combinations for the treatment and/or prevention of diseases, and to their use for preparing medicaments for the treatment and/or prevention of diseases, in particular for treatment and/or prevention of cardiovascular, renal, inflammatory and fibrotic diseases.
表征谱图
-
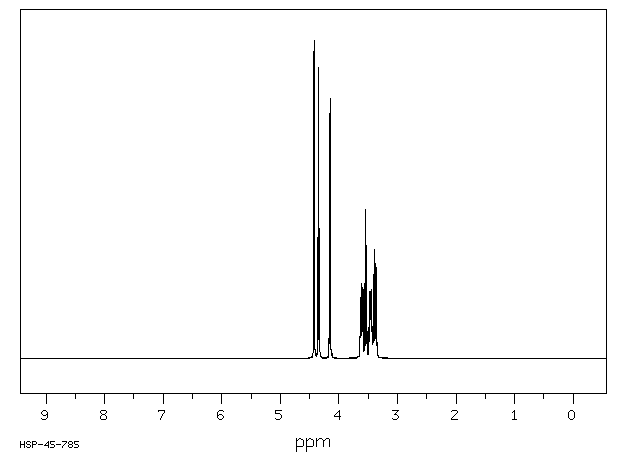
氢谱1HNMR
-
质谱MS
-
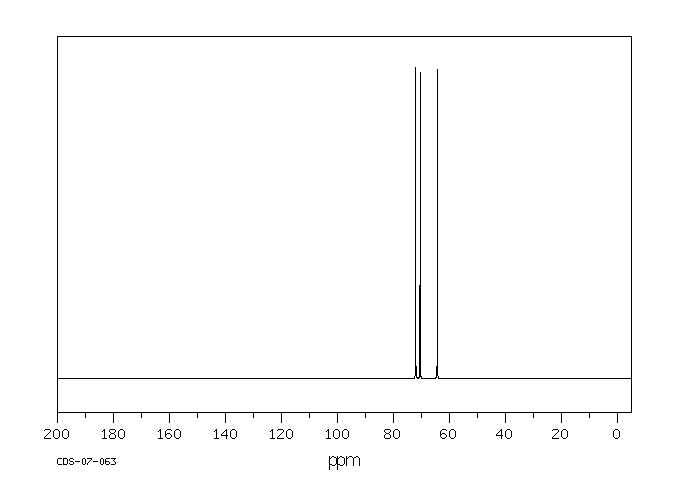
碳谱13CNMR
-
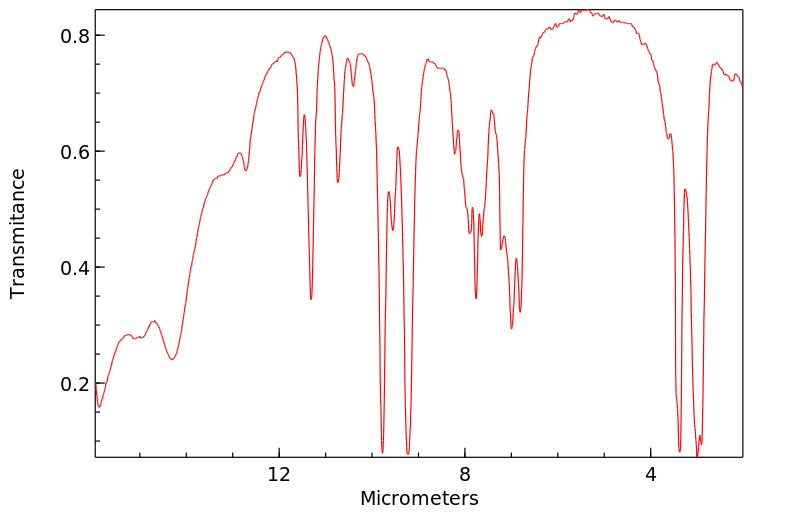
红外IR
-
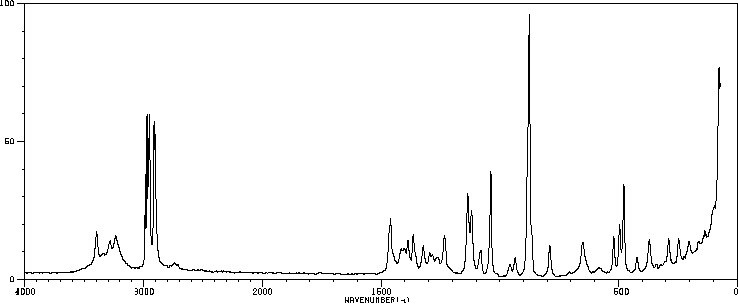
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息