D-Arabitol | 488-82-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:101-104 °C
-
比旋光度:+10~+14゜(20℃/D)(c=5,Na2B4O7 soln.)
-
沸点:194.6°C (rough estimate)
-
密度:1.1497 (rough estimate)
-
溶解度:在水中的溶解度0.1 g/mL,澄清,无色
-
LogP:-3.774 (est)
-
物理描述:Solid
-
碰撞截面:135.8 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
计算性质
-
辛醇/水分配系数(LogP):-2.5
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:101
-
氢给体数:5
-
氢受体数:5
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S24/25,S26,S36
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
海关编码:29054980
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— Xylitol 87-99-0 C5H12O5 152.147 —— Adonitol 488-81-3 C5H12O5 152.147 —— L-(-)-arabitol 7643-75-6 C5H12O5 152.147 DL-阿拉伯糖醇 1,2,3,4,5-pentahydroxy-pentane 7643-75-6 C5H12O5 152.147 山梨醇 D-sorbitol 50-70-4 C6H14O6 182.174 1L-手性纤维醇 inositol 551-72-4 C6H12O6 180.158 D-来苏糖 D-lyxose 1114-34-7 C5H10O5 150.131 D-木糖 D-xylose 58-86-6 C5H10O5 150.131 阿拉伯糖 D-Arabinose 147-81-9 C5H10O5 150.131 D-2-脱氧葡萄糖 D-2-deoxyglucose 154-17-6 C6H12O5 164.158 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Xylitol 87-99-0 C5H12O5 152.147 —— Adonitol 488-81-3 C5H12O5 152.147 DL-阿拉伯糖醇 1,2,3,4,5-pentahydroxy-pentane 7643-75-6 C5H12O5 152.147 —— D-galactitol 608-66-2 C6H14O6 182.174 甘露醇 mannitol 87-78-5 C6H14O6 182.174 —— (2S,4S)-pentane-1,2,3,4-tetraol 92691-36-6 C5H12O4 136.148 —— 3-O-methyl-D-arabinitol —— C6H14O5 166.174 阿拉伯糖 D-Arabinose 147-81-9 C5H10O5 150.131 L-来苏糖 L-lyxose 1949-78-6 C5H10O5 150.131 D-木糖 D-xylose 58-86-6 C5H10O5 150.131 —— bis[(2R)-oxiran-2-yl]methanol 63941-88-8 C5H8O3 116.117 - 1
- 2
反应信息
-
作为反应物:描述:D-Arabitol 在 咪唑 、 氯化亚砜 作用下, 以 四氢呋喃 为溶剂, 反应 0.5h, 以97%的产率得到bis[(4R)-2-oxo-1,3,2-dioxathiolan-4-yl]methanol参考文献:名称:Synthesis of α,ω‐Diazidoalditol Derivatives via Both bis‐ or tris‐Cyclic Sulfites and Peracetylated α,ω‐Dibromoalditols as Bielectrophilic Intermediates摘要:The alpha,omega-diazidoalditol derivatives with erythro, threo, xylo, ribo, D-arabino, D-manno, and D-gluco configuration were efficiently synthesized, respectively, from bis- or tris-cyclic sulfite or peracetylated alpha,omega-dibromoalditol intermediates. The cyclic sulfite intermediates has the advantage to lead directly to the free alpha,omega-diazido-alpha,omega-dideoxyalditols.DOI:10.1081/car-120030470
-
作为产物:描述:(S)-1-((4R,4'R,5R)-2,2,2',2'-tetramethyl-4,4'-bi(1,3-dioxolan)-5-yl)ethane-1,2-diol 在 sodium tetrahydroborate 、 硫酸 、 periodate 作用下, 以 甲醇 为溶剂, 反应 50.0h, 生成 D-Arabitol参考文献:名称:糖烯丙基锡与醛的反应。呋喃糖和吡喃糖有机金属衍生物之间的反应性有显着差异摘要:研究了单糖的有机金属衍生物与BF 3 ·OEt 2催化的醛类的反应。注意到吡喃硅烷基和呋喃基硅烷基烯丙基锡之间的反应性有显着差异。前者容易与醛反应,从而提供具有非常高的立体选择性的高级碳糖的前体,而后者在与醛反应之前进行了重排,消除了苯乙烯基部分。DOI:10.1016/s0957-4166(01)00331-7
-
作为试剂:描述:参考文献:名称:Process for the preparation of D-arabitol摘要:一种制备D-阿拉伯醇的工艺,其特征在于包括以下阶段:水解乳糖溶液,将所得的葡萄糖和半乳糖混合物氧化为葡萄糖酸和半乳糖酸混合物,将此葡萄糖酸和半乳糖酸混合物脱羧为D-阿拉伯糖和D-吕克糖混合物,催化氢化此D-阿拉伯糖和D-吕克糖混合物以制备D-阿拉伯醇。公开号:US05846794A1
文献信息
-
Selective C−O Bond Cleavage of Sugars with Hydrosilanes Catalyzed by Piers’ Borane Generated In Situ作者:Jianbo Zhang、Sehoon Park、Sukbok ChangDOI:10.1002/anie.201708109日期:2017.10.23[(C6F5)2BH], generated in situ, is demonstrated to promote the hydrosilylative reduction of sugars, providing a series of linear or cyclic polyols with high chemo- and regioselectivities under mild conditions. Studies of catalytic reactivity and regioselectivity with regard to the C−O bond cleavage with hydrosilanes suggest an importance of the steric environment around the anomeric carbon center of the
-
(1-13C)Alditols: elimination of magnetic equivalence in 1H-and 13C-n.m.r. spectra of symmetric compounds through (13C)-substitution作者:Eugenia C. Garrett、Anthony S. SerianniDOI:10.1016/0008-6215(90)80082-e日期:1990.12(1-13C)Glycerol, D-(1-13C)arabinitol, D-(1-13C)ribitol, D-(1-13C)xylitol, D-(1-13C)glucitol, D-(1-13C)mannitol, and D-(1-13C)talitol have been prepared by NaBH4 reduction of the corresponding (1-13C)aldoses. A comparison of the 1H- (300 and 620 MHz) and 13C (75 MHz) n.m.r. spectra of natural and (1-13C)-substituted dissymmetric alditols has permitted the unequivocal assignments of their hydroxymethyl(1-13C)甘油,D-(1-13C)阿拉伯糖醇,D-(1-13C)核糖醇,D-(1-13C)木糖醇,D-(1-13C)葡萄糖醇,D-(1-13C)甘露醇和D-(1-13C)塔罗糖醇已通过NaBH4还原相应的(1-13C)醛糖制备。比较天然和(1-13C)取代的不对称醛糖醇的1H-(300 MHz和620 MHz)和13C(75 MHz)nmr光谱,可以明确地确定其羟甲基质子和碳信号,并测量几种13C -1H和13C-13C自旋耦合常数。然而,(1-13C)取代的对称糖醇的相似光谱更难于解释,因为它们由重叠的13C耦合和13C非耦合子光谱组成。在某些情况下,可以使用1H差异光谱和1H耦合的13C光谱从13C耦合的组件中提取13C-1H和13C-13C自旋耦合。
-
Multiple Forms of Xylose Reductase in <i>Candida intermedia</i>: Comparison of Their Functional Properties Using Quantitative Structure−Activity Relationships, Steady-State Kinetic Analysis, and pH Studies作者:Bernd Nidetzky、Kaspar Brüggler、Regina Kratzer、Peter MayrDOI:10.1021/jf034426j日期:2003.12.1The xylose-fermenting yeast Candida intermedia produces two isoforms of xylose reductase: one is NADPH-dependent (monospecific xylose reductase; msXR), and another is shown here to prefer NADH approximately 4-fold over NADPH (dual specific xylose reductase; dsXR). To compare the functional properties of the isozymes, a steady-state kinetic analysis for the reaction d-xylose + NAD(P)H + H(+) <--> xylitol木糖发酵酵母假丝酵母产生两种木糖还原酶同工型:一种是NADPH依赖性的(单特异性木糖还原酶; msXR),另一种是NADH优于NADPH(双特异性木糖还原酶; dsXR)的4倍。为了比较同工酶的功能特性,对反应中的d-木糖+ NAD(P)H + H(+)<->木糖醇+ NAD(P)(+)进行了稳态动力学分析,并进行了特异性分析测定一系列常数(k(cat)/ K(醛))的常数,以减少一系列侧链大小不同的醛的还原以及与酶的底物结合口袋的氢键结合能力。dsXR弱结合NAD(P)(+)(K(iNAD +)= 70 microM; K(iNADP +)= 55 microM)和NADH(K(i)= 8 microM)大约与NADPH(K(i)= 14 microM)。msXR显示NADPH和NADP(+)的均匀结合(K(iNADP +)大约为K(iNADPH)= 20 microM)。通过将dsXR的对数k(cat)/
-
Conversion of sugars to ethylene glycol with nickel tungsten carbide in a fed-batch reactor: high productivity and reaction network elucidation作者:Roselinde Ooms、Michiel Dusselier、Jan A. Geboers、Beau Op de Beeck、Rick Verhaeven、Elena Gobechiya、Johan A. Martens、Andreas Redl、Bert F. SelsDOI:10.1039/c3gc41431k日期:——Bifunctional nickel tungsten carbide catalysis was used for the conversion of aqueous sugar solutions into short-chain polyols such as ethylene glycol. It is shown that very concentrated sugar solutions, viz. up to 0.2 kg L−1, can be converted without loss of ethylene glycol selectivity by gradually feeding the sugar solution. Detailed investigation of the reaction network shows that, under the applied reaction conditions, glucose is converted via a retro-aldol reaction into glycol aldehyde, which is further transformed into ethylene glycol by hydrogenation. The main byproducts are sorbitol, erythritol, glycerol and 1,2-propanediol. They are formed through a series of unwanted side reactions including hydrogenation, isomerisation, hydrogenolysis and dehydration. Hydrogenolysis of sorbitol is only a minor source of ethylene glycol. To assess the relevance of the fed-batch system in biomass conversions, both the influence of the catalyst composition and the reactor setup parameters like temperature, pressure and glucose addition rate were optimized, culminating in ethylene glycol yields up to 66% and separately, volume productivities of nearly 300 gEG L−1 h−1.双功能镍钨碳化物催化剂用于将水溶性糖溶液转化为乙二醇等短链多元醇。结果表明,通过逐渐加入糖溶液,可以转化非常高浓度的糖溶液(高达0.2 kg/L),而不会损失乙二醇的选择性。详细研究反应网络显示,在应用的反应条件下,葡萄糖通过逆醛醇反应转化为甘油醛,甘油醛进一步通过氢化反应转化为乙二醇。主要副产物是山梨醇、赤藓糖醇、甘油和1,2-丙二醇。它们是通过一系列不希望的副反应(包括氢化、异构化、氢解和脱水)形成的。山梨醇的氢解只是乙二醇的次要来源。为了评估批量进料系统在生物质转化中的相关性,优化了催化剂组成和反应器设置参数(如温度、压力和葡萄糖添加速率),最终乙二醇的收率高达66%,体积产率接近300 gEG/L/h。
-
Preparation of enantiomeric and racemic 2,3,4,5-tetrahydroxypentyl derivatives of adenine, cytosine and uracil作者:Antonín HolýDOI:10.1135/cccc19822786日期:——
1-(Adenin-9-yl)-1-deoxy-DL-ribitol (
III ), -D-arabitol (IXa ), -L-arabitol (XIVa ), -DL-xylitol (XXIVa ), 1-(cytosin-L-yl)-1-deoxy-D-arabitol (IXb ), -L-arabitol (XIVb ), 1-(uracil-1-yl)-1-deoxy-D-arabitol (IXc ), -L-arabitol (XIVc ) and -DL-xylitol (XXIVb ) were prepared by reaction of 1-O-p -toluenesulfonyl-2,3:4,5-di-O-isopropylidenealditolsIb, VIIb, XIIb andXXIIb with sodium salts of adenine, N4-benzoylcytosine or 4-methoxy-2-pyrimidone followed by removal of the protecting groups. Condensation of the mentioned sodium salts with methyl 5-O-p -toluenesulfonyl-2,3-O-isopropylidene-β-D-ribofuranoside (IV ) with subsequent acid hydrolysis and reduction with sodium borohydride afforded 1-(adenin-9-yl)-1-deoxy-L-ribitol (VIa ) and 1-(cytosin-1-yl)-1-deoxy-L-ribitol (VIb ). 1-(Adenin-9-yl)-1-deoxy-L-lyxitol (XVII ), -L-lyxitol (XVIII ) and -2-O-methyl-D-lyxitol (XXI ) were prepared analogously. Acid hydrolysis of 5-(adenin-9-yl)-5-deoxy-4-O-benzyl-1,2-O-isopropylidene-α-D-xylofuranose (XXVa ), followed by reduction with sodium borohydride and catalytic hydrogenation, gave 1-(adenin-9-yl)-1-deoxy-L-xylitol (XXVIb ).1-(腺嘌呤-9-基)-1-脱氧-DL-核糖醇(III),-D-阿拉伯糖醇(IXa),-L-阿拉伯糖醇(XIVa),-DL-木糖醇(XXIVa),1-(胞嘧啶-L-基)-1-脱氧-D-阿拉伯糖醇(IXb),-L-阿拉伯糖醇(XIVb),1-(尿嘧啶-1-基)-1-脱氧-D-阿拉伯糖醇(IXc),-L-阿拉伯糖醇(XIVc)和-DL-木糖醇(XXIVb)通过将1-O-p-甲苯磺酰基-2,3:4,5-二-O-异丙基亚甲基二醇(Ib,VIIb,XIIb)和(XXIIb)与腺嘌呤、N4-苯甲酰胞嘧啶或4-甲氧基-2-嘧啶酮的钠盐反应制备,随后去除保护基。提到的钠盐与甲基5-O-p-甲苯磺酰基-2,3-O-异丙基亚-D-核糖呋喃苷(IV)缩合,随后进行酸水解和硼氢化钠还原,得到1-(腺嘌呤-9-基)-1-脱氧-L-核糖醇(VIa)和1-(胞嘧啶-1-基)-1-脱氧-L-核糖醇(VIb)。1-(腺嘌呤-9-基)-1-脱氧-L-利克糖醇(XVII),-L-利克糖醇(XVIII)和-2-O-甲基-D-利克糖醇(XXI)类似制备。5-(腺嘌呤-9-基)-5-脱氧-4-O-苄基-1,2-O-异丙基亚-D-木糖呋喃糖醇(XXVa)的酸水解,随后与硼氢化钠还原和催化氢化,得到1-(腺嘌呤-9-基)-1-脱氧-L-木糖醇(XXVIb)。
表征谱图
-
氢谱1HNMR
-
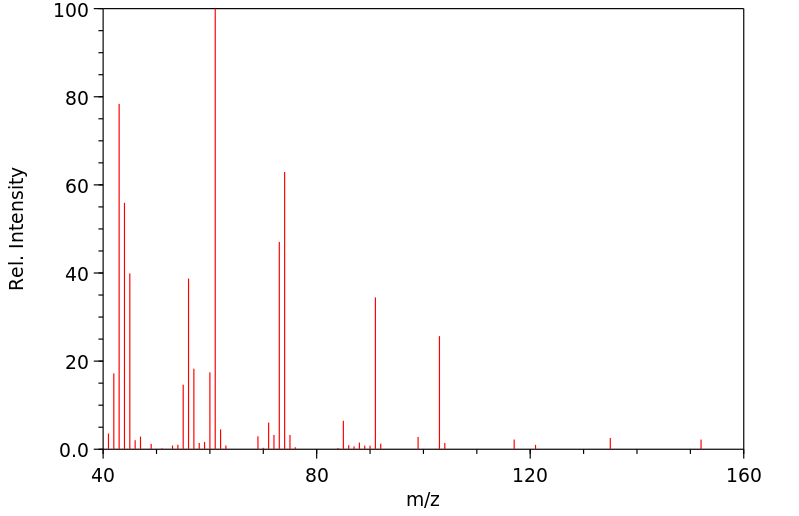
质谱MS
-
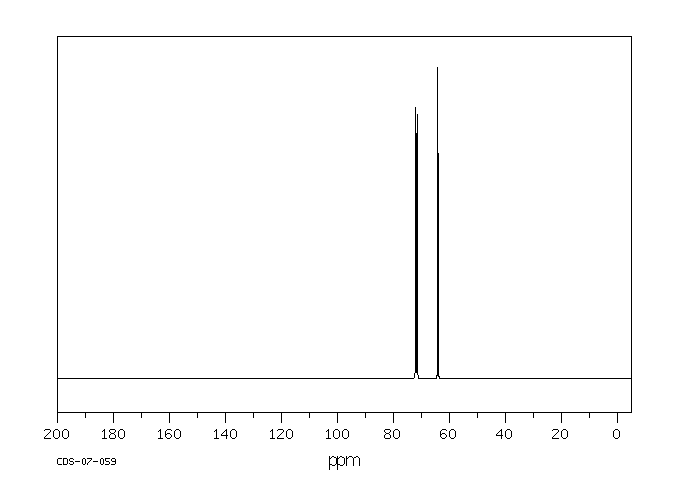
碳谱13CNMR
-
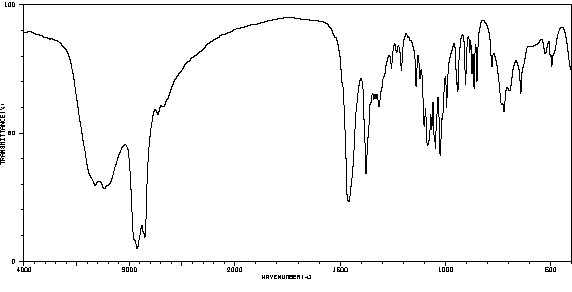
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息