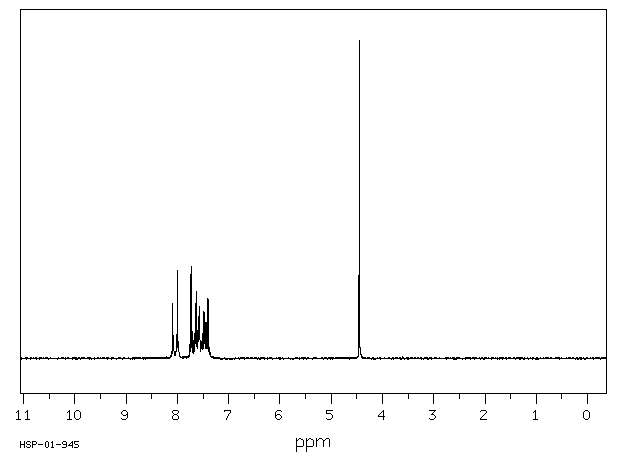
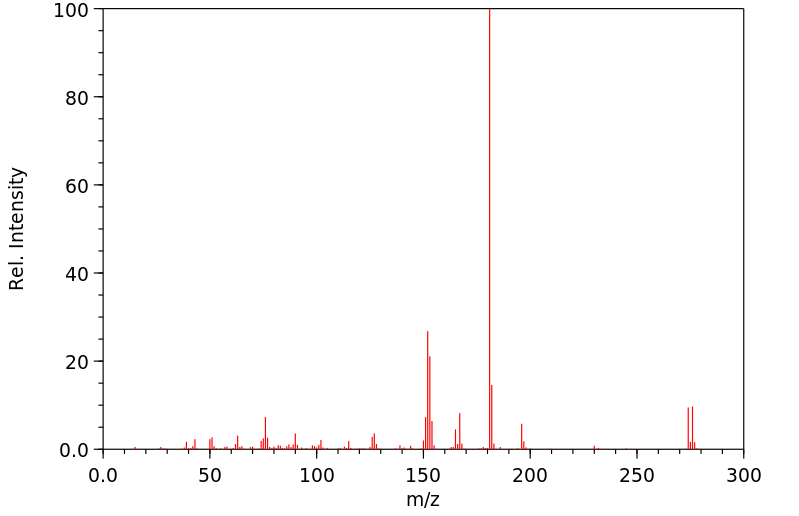
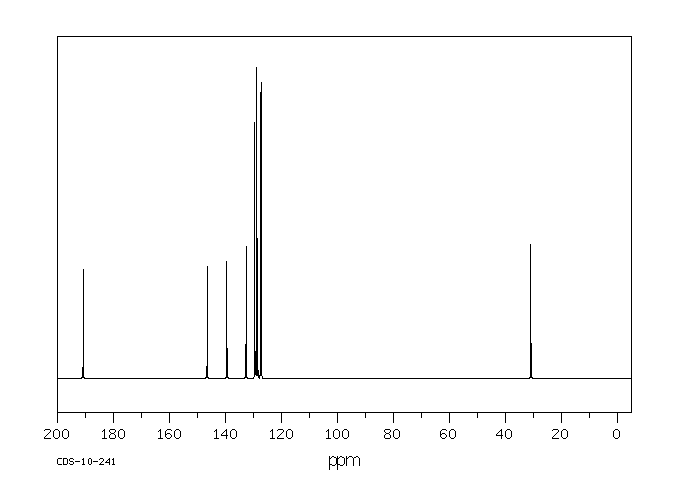
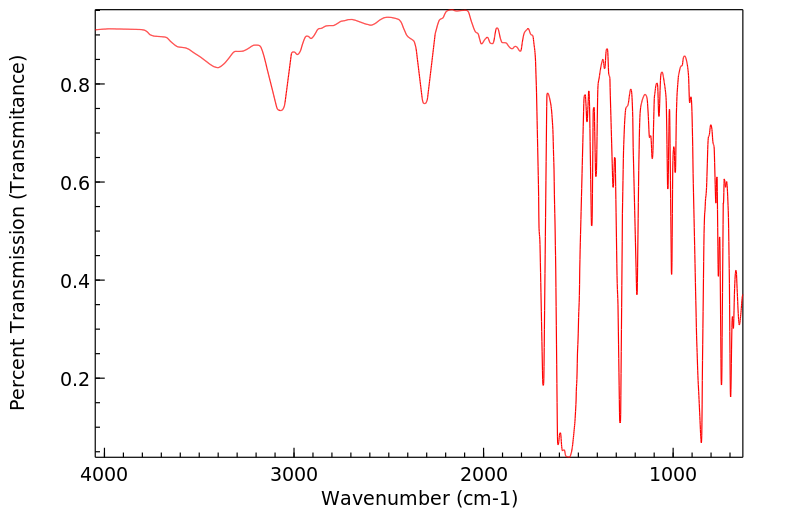
The normally robust monoalkylated complexes [Pt2(µ-S)(µ-SR)(PPh3)4]+ can be activated towards further alkylation. Dialkylated complexes [Pt2(µ-SR)2(PâP)2]2+ (PâP = 2 à PPh3, Ph2P(CH2)3PPh2) can be stabilized and isolated by the use of electron-rich and aromatic halogenated substituents R [e.g. 3-(2-bromoethyl)indole and 2-bromo-4â²-phenylacetophenone] and 1,3-bis(diphenylphosphino)propane [Ph2P(CH2)3PPh2 or dppp] which enhances the nucleophilicity of the Pt2(µ-S)2} core. This strategy led to the activation of [Pt2(µ-S)(µ-SR)(PPh3)4]+ towards RâX as well as isolation and crystallographic elucidation of [Pt2(µ-SC10H10N)2(PPh3)4](PF6)2 (2a), [Pt2(µ-SCH2C(O)C6H4C6H5)2(PPh3)4](PF6)2 (2b), and a range of functionalized-thiolato bridged complexes such as [Pt2(µ-SR)2(dppp)2](PF6)2 [R = âCH2C6H5 (8a), âCH2CHCH2 (8b) and âCH2CN (8c)]. The stepwise alkylation process is conveniently monitored by Electrospray Ionisation Mass Spectrometry, allowing for a direct qualitative comparison of the nucleophilicity of [Pt2(µ-S)2(PâP)2], thereby guiding the bench-top synthesis of some products observed spectroscopically.
通常情况下,强健的单烷基化配合物 [Pt2(µ-S)(µ-SR)(PPh3)4]+ 可以被进一步活化进行烷基化。通过使用电富荷和芳香性卤代取代基 R(例如
3-(2-溴乙基)吲哚和 2-
溴-4'-苯基
乙酰苯酮)以及提高 Pt2(µ-S)2} 核的亲核性的 1,3-双(
二苯基膦基)
丙烷 [Ph2P(
CH2)3PPh2 或 dppp],可以稳定和分离二烷基化配合物 [Pt2(µ-SR)2(PâP)2]2+ (PâP = 2 ×
磷三苯,Ph2P( )3PPh2)。这一策略不仅激活了 [Pt2(µ-S)(µ-SR)(PPh3)4]+ 对 R-X 的反应性,还成功分离和晶体结构解析了 [Pt2(µ-SC10H10N)2(PPh3)4](PF6)2 (2a)、[Pt2(µ-S C(O)C6H4C6H5)2(PPh3)4](PF6)2 (2b) 以及一系列功能化
硫醇基桥联配合物,如 [Pt2(µ-SR)2(dppp)2](PF6)2 [R = - C6H5 (8a)、- CH (8b) 和 - CN (8c)]。通过电喷雾离子化质谱法可以方便地监测逐步烷基化过程,从而直接定性比较 [Pt2(µ-S)2(PâP)2] 的亲核性,指导某些光谱观察到的产品的实验台合成。