辛可尼丁 | 485-71-2
中文名称
辛可尼丁
中文别名
辛可尼定;(9S)-6'-甲氧基辛可宁-9-醇;类金鸡纳碱;金鸡尼丁;辛可尼啶;4-喹啉基-(5-乙烯基-1-氮杂双环[2.2.2]辛烷-2-基)甲醇;(9S)-6"-甲氧基辛可宁-9-醇
英文名称
Cinchonidin
英文别名
Cinchonidine;(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-quinolin-4-ylmethanol
CAS
485-71-2
化学式
C19H22N2O
mdl
——
分子量
294.396
InChiKey
KMPWYEUPVWOPIM-KODHJQJWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204-206 °C(lit.)
-
比旋光度:-115 º (c=1, EtOH)
-
沸点:436.16°C (rough estimate)
-
密度:1.0863 (rough estimate)
-
溶解度:0.25克/升
-
LogP:2.68 at 25℃
-
碰撞截面:168.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:22
-
可旋转键数:3
-
环数:5.0
-
sp3杂化的碳原子比例:0.42
-
拓扑面积:36.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S36/37
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2939200000
-
包装等级:III
-
危险类别:6.1(b)
-
危险品运输编号:UN 1544
-
储存条件:密封保存,并置于通风、干燥的地方,避免与氧化物接触。
SDS
| Name: | Cinchonidine 98.5-101% (On The Dried Substance) Material Safety Data Sheet |
| Synonym: | Alpha-Quinidine |
| CAS: | 485-71-2 |
Synonym:Alpha-Quinidine
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 485-71-2 | Cinchonidine | 98% | 207-622-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Light sensitive.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Store protected from light.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from light.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 485-71-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white to cream
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 200.00 - 203.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: practically insoluble in water
Specific Gravity/Density:
Molecular Formula: C19H22N2O
Molecular Weight: 294.39
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, light, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 485-71-2: GD2975000 LD50/LC50:
Not available.
Carcinogenicity:
Cinchonidine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 485-71-2: 2
Canada
CAS# 485-71-2 is listed on Canada's NDSL List.
CAS# 485-71-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 485-71-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
简介
辛可尼丁是一种喹啉型生物碱,属于金鸡纳生物碱的一种,又称金鸡尼丁,是辛可宁的立体异构体。它在醚中结晶为白色针状或棱形晶体,熔点为204~206℃,比旋光度-115°(乙醇,C=1),折射率-107.5°(乙醇,C=1)。辛可尼丁能溶于醇、醚、丙酮、苯和氯仿,几乎不溶于冷水。
应用辛可尼丁作为有机合成中间体和医药中间体,在实验室有机合成过程中和化工医药研发中被广泛应用。
作用辛可尼丁与奎宁相似,具有抗疟疾的作用。在从金鸡纳树皮提取液中分离出奎宁后,可以通过碱处理母液,并加入酒石酸使其生成难溶的酒石酸盐来分离出生物碱。
结构特点金鸡纳碱(如奎宁、奎尼丁、辛可宁和辛可尼丁等)是一类在约两个世纪前从金鸡纳树皮中发现的重要天然产物。在过去,它们是治疗疟疾的特效药;近年来,在不对称合成中作为催化剂的优势骨架得到了广泛认可。从生物合成的角度来看,金鸡纳生物碱是由单萜吲哚生物碱衍生而来。
生物活性 靶点 体外研究上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 辛可宁 Cinchonin 118-10-5 C19H22N2O 294.396 —— 4-[[(2R,4R,5S)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]methyl]quinoline —— C19H22N2 278.397 —— cinchonidine diphenylmethylsilyl ether —— C32H34N2OSi 490.72 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 9-表-辛可尼丁 (1S,3R,4S,8S,9S)-9-hydroxy-cinchonane 550-54-9 C19H22N2O 294.396 辛可宁 Cinchonin 118-10-5 C19H22N2O 294.396 (8alpha,9R)-10,11-二氢脱氧辛可宁-9-醇 (-)-hydrocinchonidine 485-64-3 C19H24N2O 296.412 —— 11-hydroxymethyl-10,11-dihydrocinchonidine —— C20H26N2O2 326.439 —— O-methylcinchonidine —— C20H24N2O 308.423 —— 10,11-dihydrocinchonidine-11-carbaldehyde 445433-05-6 C20H24N2O2 324.423 —— 11-N-benzylaminomethyl-10,11-dihydrocinchonidine —— C27H33N3O 415.579 —— O-ethyl-cinchonidine —— C21H26N2O 322.45 —— 11-(4-(trifluoromethyl)phenyl)cinchonidine —— C26H25F3N2O 438.493 —— 11-(biphenyl-4-yl)cinchonidine —— C31H30N2O 446.592 —— 11-(triethylsilyl)-10,11-dihydrocinchonidine —— C25H38N2OSi 410.675 —— 11-(4-(ethylcarboxy)phenyl)cinchonidine —— C28H30N2O3 442.558 —— 11-(3-(trifluoromethyl)phenyl)cinchonidine —— C26H25F3N2O 438.493 —— cinchonidine O-(9)-allyl ether 833480-02-7 C22H26N2O 334.461 —— (R)-((1S,2S,4S,5R)-5-{3-[(Z)-Benzylimino]-propyl}-1-aza-bicyclo[2.2.2]oct-2-yl)-quinolin-4-yl-methanol 445433-08-9 C27H31N3O 413.563 —— 4-[(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-[[4-[[(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-quinolin-4-ylmethoxy]methyl]phenyl]methoxy]methyl]quinoline 1430743-66-0 C46H50N4O2 690.929 —— 1,2-bis[(10,11-dihydrocinchonidin-11-yl)dimethylsilyl]ethane —— C44H62N4O2Si2 735.173 —— 9-O-(trimethylsilyl)cinchonidine 518028-05-2 C22H30N2OSi 366.578 —— acetylcinchonidine —— C21H24N2O2 336.434 —— 9-Acetoxy-cinchonan —— C21H24N2O2 336.434 —— 11-(triethoxysilyl)-10,11-dihydrocinchonidine —— C25H38N2O4Si 458.673 —— O-phenylcinchonidine —— C25H26N2O 370.494 —— (8S,9R)-9-O-mesylcinchonidine 560119-42-8 C20H24N2O3S 372.488 —— Deoxycinchonidine 5808-37-7 C19H22N2 278.397 —— [(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-quinolin-4-ylmethyl] benzoate —— C26H26N2O2 398.505 —— [(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-quinolin-4-ylmethyl] 4-methylbenzoate 500553-17-3 C27H28N2O2 412.532 —— 4-[(R)-(tert-Butyl-dimethyl-silanyloxy)-((1S,2S,4S,5R)-5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl)-methyl]-quinoline 183994-23-2 C25H36N2OSi 408.659 —— [(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-quinolin-4-ylmethyl] 2-phenylacetate 500553-20-8 C27H28N2O2 412.532 —— cinchonidine —— C42H60N2O3 640.95 —— 11-(triphenylsilyl)-10,11-dihydrocinchonidine —— C37H38N2OSi 554.807 —— [(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-quinolin-4-ylmethyl] 4-methoxybenzoate —— C27H28N2O3 428.531 —— [(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-quinolin-4-ylmethoxy]-diphenylphosphane 76116-16-0 C31H31N2OP 478.574 —— cinchonine —— C44H62N2O3 666.988 —— 2-((pyridin-2-yloxy)(quinolin-4-yl)methyl)-8-vinylquinuclidine —— C24H25N3O 371.482 —— 11-(triethylsilyl)-9-O-(trimethylsilyl)-10,11-dihydrocinchonidine 863332-65-4 C28H46N2OSi2 482.857 —— 9-Salicyloyloxy-cinchonan —— C26H26N2O3 414.504 —— 4-[(R)-[(4S,5S)-4,5-diphenyl-1,3,2-dioxaphospholan-2-yl]oxy-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]methyl]quinoline 1108188-23-3 C33H33N2O3P 536.61 —— 1,2-bis{[9-O-(trimethylsilyl)-10,11-dihydrocinchonidin-11-yl]dimethylsilyl}ethane 863332-68-7 C50H78N4O2Si4 879.537 —— cinchonidine —— C46H64N2O5 725.025 —— [(CN)PYDZ] 1094424-49-3 C23H23ClN4O 406.915 —— 9-O-(trimethylsilyl)-11-(triethoxysilyl)-10,11-dihydrocinchonidine —— C28H46N2O4Si2 530.855 9-氨基-(9-脱氧)表辛可尼丁三盐酸盐 9-amino(9-deoxy)epicinchonidine 850409-61-9 C19H23N3 293.412 —— (8R,9R)-9-amino(9-desoxy)-epi-cinchonine 779974-91-3 C19H23N3 293.412 —— cinchoninone —— C19H20N2O 292.381 —— Cinchonan-9-al —— C19H20N2O 292.381 —— Chinchonidinon —— C19H20N2O 292.381 —— (3R,4S,8S,9R)-9-fluorocinchonane 691382-02-2 C19H21FN2 296.388 —— 11-(triphenylsilyl)-9-O-(trimethylsilyl)-10,11-dihydrocinchonidine 863332-66-5 C40H46N2OSi2 626.989 —— cinchonidine diphenylmethylsilyl ether —— C32H34N2OSi 490.72 —— O-((R)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)(3,5-bis(trifluoromethyl)phenyl)carbamothioate —— C29H27F6N3O2S 595.609 (8Α,9S)-10,11-二氢奎宁-9-胺 (S)-((1S,2S,4S,5R)-5-ethylquinuclidin-2-yl)(quinolin-4-yl)methanamine 1253690-81-1 C19H25N3 295.428 —— [(CN)PHAL] 1094424-43-7 C27H25ClN4O 456.975 —— (1S,3R,4S,8S,9S)-9-phenylsulfanylcinchonan —— C25H26N2S 386.561 —— (1S,3R,4S,8S,9R)-9-phenylsulfanylcinchonan —— C25H26N2S 386.561 —— (8S,9S)-9-L-leucinylamide(9-deoxy)-epi-cinchonidine 1640010-92-9 C25H34N4O 406.571 —— 1-phenyl-3-((S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)thiourea —— C26H28N4S 428.601 —— (8S,9S)-9-L-prolinylamide(9-deoxy)-epi-cinchonidine 1040729-03-0 C24H30N4O 390.528 N-(9-脱氧-epi-辛克宁-9-基)氮苯酰胺 (8S,9S)-9-picolinamide(9-desoxy)-epi-cinchonidine 1414851-57-2 C25H26N4O 398.508 普瑞巴林杂质D epiCDT 890044-38-9 C28H26F6N4S 564.598 N-[3,5-双(三氟甲基)苯基]-N'-(8Α,9S)-奎宁-9-基脲 epi-cinchonidine 957770-67-1 C28H26F6N4O 548.531 1-(4-喹啉基)-3-[(3R,4R)-3-乙烯基-4-哌啶基]-1-丙酮 cinchonicine 69-24-9 C19H22N2O 294.396 —— 1-quinolin-4-yl-3-(vinyl-piperidin-4-yl)-propan-1-one 344871-97-2 C19H22N2O 294.396 —— 3-ethoxy-2-(quinolin-4-yl)-6-vinyl-1-azabicyclo[3.2.2]nonane —— C21H26N2O 322.45 —— (2R,3S,5S,6R)-3-fluoro-2-(quinolin-4'-yl)-6-vinyl-1-azabicyclo[3.2.2]nonane 691381-89-2 C19H21FN2 296.388 —— (2S,3R,5S,6R)-3-fluoro-2-(quinolin-4'-yl)-6-vinyl-1-azabicyclo[3.2.2]nonane 691381-91-6 C19H21FN2 296.388 —— (S)-N-8-Quinolinesulfonyl-(quinolin-4-yl)(8-vinylquinuclidin-2-yl)methanamine —— C28H28N4O2S 484.622 —— quinine 1256245-86-9 C31H26F6N4O2 600.563 - 1
- 2
- 3
- 4
- 5
- 6
- 7
反应信息
-
作为反应物:描述:参考文献:名称:对映选择性相转移催化的1-甲基-7-甲氧基-2-四氢萘酮的烷基化:脱佐辛的有效途径。摘要:为了不对称地制备(R)-(+)-1-(5-溴戊基)-1-甲基-7-甲氧基-2-四氢萘酮(3a),制备了地佐辛的关键中间体17金鸡纳生物碱衍生催化剂并用1,5-二溴戊烷筛选1-甲基-7-甲氧基-2-四氢萘酮的对映选择性烷基化反应,并确定了最佳催化剂(C7)。另外,对烷基化进行了优化,使得该方法变得实用和有效。DOI:10.3762/bjoc.14.119
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 生成 辛可尼丁参考文献:名称:102.将氢奎尼丁转化为氢辛可宁,将铜脲转化为辛可尼定摘要:DOI:10.1039/jr9460000523
-
作为试剂:描述:3-烯丙基-2-羟基苯甲酸甲酯 在 氯化亚砜 、 三乙胺 、 辛可尼丁 作用下, 以 异丙醇 、 苯 为溶剂, 反应 6.0h, 生成 (S)-4-(2-methyl-2,3-dihydro-7-benzofuroyl)amino-1-methyl-3-propyl-5-pyrazolecarboxamide参考文献:名称:Synthesis and Vasorelaxant Potency of Monagra. A Chiral 5-(2-Methyl-2,3-dihydro-7-benzofuryl)pyrazolopyrimidone Analog of Viagra®摘要:DOI:10.3987/com-01-9218
文献信息
-
Synthesis of novel cinchona-amino acid hybrid organocatalysts for asymmetric catalysis作者:Pedro Barrulas、Maurizio Benaglia、Anthony J. BurkeDOI:10.1016/j.tetasy.2014.05.003日期:2014.6Three novel subclasses of cinchonidine derivatives coupled to diverse amino acids were prepared in very good overall yield and tested in a benchmark organocatalytic aldol reaction, between acetone and aromatic aldehydes. These subclasses are a family of amino acid-cinchonidine (subclass A), N-formamides-cinchonidine (subclass B) and dipeptide-cinchonidine (subclass C) hybrids. Our main goal, besides
-
[EN] INDAZOLE- AND PYRROLOPYRIDINE-DERIVATIVE AND PHARMACEUTICAL USE THEREOF<br/>[FR] DÉRIVÉ D'INDAZOLE ET PYRROLOPYRIDINE ET UTILISATION PHARMACEUTIQUE DE CELUI-CI申请人:DAINIPPON SUMITOMO PHARMA CO公开号:WO2012169649A1公开(公告)日:2012-12-13The present invention relates to a novel indazole- or pyrrolopyridine-derivative, represented by the formula (1) below, that has an agonistic action or a partial agonistic action against serotonin-4 receptor, and a pharmaceutical composition comprising the same. Formula (1) [wherein each substituent is as defined in claim 1]
-
FIBROSIS INHIBITOR申请人:Sumitomo Pharmaceuticals Company, Limited公开号:EP1479384A1公开(公告)日:2004-11-24Medicament being useful as a fibrosis inhibitor for organs or tissues, which comprises a compound of the formula (I): wherein Ring Z is optionally substituted pyrrole ring, etc.; W2 is -CO-, -SO2-, optionally substituted C1-C4 alkylene, etc.; Ar2 is optionally substituted aryl, etc.; W1 and Ar1 mean the following (1) and (2): (1) W1 is optionally substituted C1-C4 alkylene, etc.; Ar1 is optionally substituted bicyclic heteroaryl having 1 to 4 nitrogen atoms as ring-forming atoms: (2) W1 is optionally substituted C2-C5 alkylene, optionally substituted C2-C5 alkenylene, etc.; and Ar1 is aryl or monocyclic heteroaryl, which is substituted by carboxyl, alkoxycarbonyl, etc. at the ortho- or meta-position thereof with respect to the binding position of W1, or a pharmaceutically acceptable salt thereof.
-
SILICON BASED DRUG CONJUGATES AND METHODS OF USING SAME申请人:BlinkBio, Inc.公开号:US20170202970A1公开(公告)日:2017-07-20Described herein are silicon based conjugates capable of delivering one or more payload moieties to a target cell or tissue. Contemplated conjugates may include a silicon-heteroatom core, one or more optional catalytic moieties, a targeting moiety that permits accumulation of the conjugate within a target cell or tissue, one or more payload moieties (e.g., a therapeutic agent or imaging agent), and two or more non-interfering moieties covalently bound to the silicon-heteroatom core.
-
[EN] HETEROBICYCLIC COMPOUNDS FOR INHIBITING THE ACTIVITY OF SHP2<br/>[FR] COMPOSÉS HÉTÉROBICYCLIQUES POUR INHIBER L'ACTIVITÉ DE SHP2申请人:TAIHO PHARMACEUTICAL CO LTD公开号:WO2020022323A1公开(公告)日:2020-01-30A compound of formula (I):wherein Ring A, Q, R1,R2, R3, R4, R5, R6, R7, R8, R9, R10, R11,X, a,b, c and d are as defined in the specification.其中环A,Q,R1,R2,R3,R4,R5,R6,R7,R8,R9,R10,R11,X,a,b,c和d的化合物的化学式(I):在规范中定义。
表征谱图
-
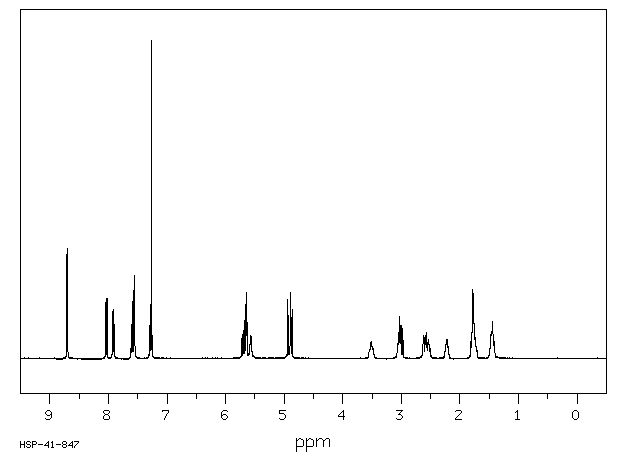
氢谱1HNMR
-
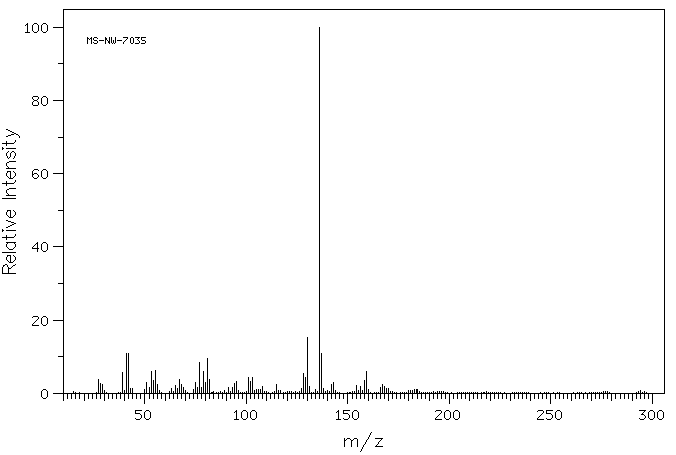
质谱MS
-
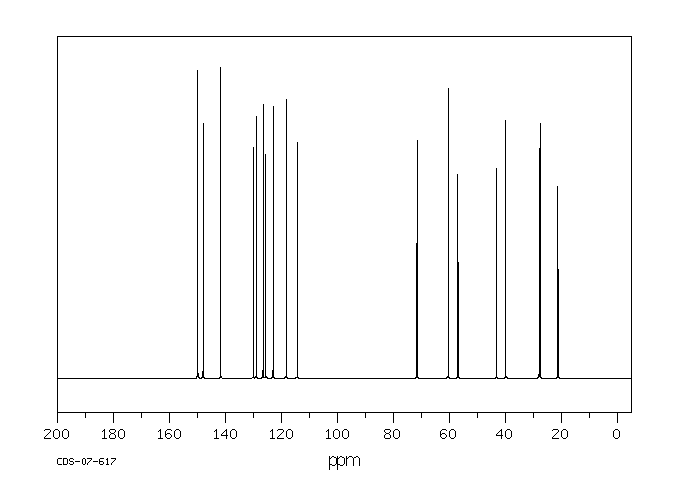
碳谱13CNMR
-
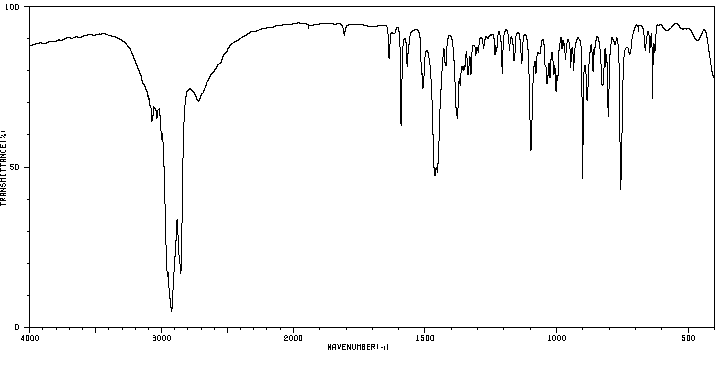
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
铜色树碱
辛可尼定硫酸盐二水合物
辛可尼定二盐酸盐
辛可尼丁盐酸盐
辛可尼丁盐酸盐
辛可尼丁
辛可宁盐酸盐
辛可宁盐酸盐
辛可宁一盐酸盐水合物
辛可宁
辉铋矿
表奎宁定
葡萄糖酸奎尼丁
苯巴比妥奎尼丁
磷酸二氢唑酯
硫酸奎尼丁二水合物
盐酸奎宁
甲酸,2-羟基丙酸,(6-甲氧基-4-喹啉基)-(5-乙烯基奎宁环-2-基)甲醇
無味奎寧
氢鲲盐酸盐
氢醌氢溴酸盐水合物
氢溴化奎宁
氢喹啉 9-菲基醚
氢化辛可宁
氢化奎尼定14-二氮杂萘二基醚混合物
氢化奎尼定 4-甲基-2-喹啉甲醚
氢化奎尼定 1,4-(2,3-二氮杂萘)二醚
氢化奎尼定
氢化奎宁4-甲基-2-喹啉甲醚
氢化奎宁-9-菲基醚
氢化奎宁(蒽醌-1,4-二基)二醚
氢化奎宁 1,4-(2,3-二氮杂萘)二醚
氢化奎宁
戊酸奎寧
弱金鸡纳碱硫酸
尤普罗辛
奎尼定盐酸盐
奎尼丁阿拉伯糖半乳聚糖硫酸酯
奎尼丁苯巴比妥
奎尼丁硫酸盐
奎尼丁甲碘化物
奎尼丁二盐酸盐
奎尼丁N-氧化物
奎尼丁
奎寧甲酸鹽
奎宁葡萄糖酸酯
奎宁苯甲酸盐
奎宁磷酸盐
奎宁磷酸盐
奎宁硫酸盐