2-氯-5-硝基苯甲酸 | 2516-96-3
中文名称
2-氯-5-硝基苯甲酸
中文别名
5-硝基邻氯苯甲酸;美沙拉嗪杂质M
英文名称
2-chloro-5-nitrobenzoic acid
英文别名
5-nitro-2-chlorobenzoic acid;2-carboxy-4-nitro-chlorobenzene
CAS
2516-96-3
化学式
C7H4ClNO4
mdl
MFCD00007294
分子量
201.566
InChiKey
QUEKGYQTRJVEQC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:165-168 °C (lit.)
-
沸点:356.5±27.0 °C(Predicted)
-
密度:1.6
-
闪点:>100°C
-
溶解度:3.6克/升
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:83.1
-
氢给体数:1
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25,S26,S39,S61
-
危险类别码:R36/37/38
-
WGK Germany:1
-
海关编码:2916399090
-
危险品运输编号:UN3077 9/PG 3
-
危险性防范说明:P261,P301+P312,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将容器密封,并将其存放在紧密封装的储存器中。应将其储存在阴凉、干燥的地方。
SDS
| Name: | 2-Chloro-5-nitrobenzoic acid 99+%(gc) Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2516-96-3 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2516-96-3 | 2-Chloro-5-nitrobenzoic acid | 99+(GC) | 219-739-7 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2516-96-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: off-white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 166.00 - 168.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water: insoluble
Specific Gravity/Density:
Molecular Formula: C7H4ClNO4
Molecular Weight: 201.57
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2516-96-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Chloro-5-nitrobenzoic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2516-96-3: 1
Canada
CAS# 2516-96-3 is listed on Canada's NDSL List.
CAS# 2516-96-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2516-96-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
概述
现有技术中制备2-氯-5-硝基苯甲酸的方法是采用邻氯苯甲酸进行硝化后再精制。但由于在硝化过程中会生成较多的2-氯-3-硝基苯甲酸异构体,常规的精制方法难以达到高纯度,一般只能控制在99.0%左右,无法满足使用需求。
应用2-氯-5-硝基苯甲酸常温常压下为浅黄色至米色棕色固体粉末状,可用作有机合成和医药化学中间体,在农药分子和生物活性分子的制备以及精细化工生产中有着较为广泛的应用。
化学性质从水中析出针状结晶。熔点在165-166℃(有时范围为166-168℃),相对密度1.608(温度条件为18/4℃)。它溶于乙醇、乙醚和苯,微溶于冷水。
用途2-氯-5-硝基苯甲酸主要用于合成2-氯-5-氨基苯甲酸、5-硝基水杨酸及其酯类和酰胺类中间体。此外,它还可作为农药、医药以及有机颜料的中间体。
生产方法由邻氯苯甲酸经硝化而得。具体步骤为:将邻氯苯甲酸与硫酸冷却至0℃以下后,加入混酸,在0℃下进行硝化反应8小时,随后加热至60℃,再倒入冰水中析出硝基物,并通过重结晶得到成品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氯-5-硝基苯甲醛 2-chloro-5-nitrobenzaldehyde 6361-21-3 C7H4ClNO3 185.567 2-氯-5-硝基苯甲酰氯 2-chloro-5-nitrobenzoylchloride 25784-91-2 C7H3Cl2NO3 220.012 2-氯-5-硝基苯乙酮 2'-chloro-5'-nitroacetophenone 23082-50-0 C8H6ClNO3 199.594 2-氯-5-硝基苯甲酰胺 2-chloro-5-nitro-benzamide 16588-15-1 C7H5ClN2O3 200.581 2-氯-5-硝基甲苯 1-chloro-2-methyl-4-nitrobenzene 13290-74-9 C7H6ClNO2 171.583 2-甲基-5-硝基苯甲酸 5-nitro-o-toluic acid 1975-52-6 C8H7NO4 181.148 5-硝基水杨酸 5-Nitrosalicylic acid 96-97-9 C7H5NO5 183.12 2-氯-5-硝基苯甲腈 4-chloro-3-cyanonitrobenzene 16588-02-6 C7H3ClN2O2 182.566 —— 2-Chlor-5-nitro-benzotrichlorid 831-50-5 C7H3Cl4NO2 274.918 —— 2-chloro-N-(4-chlorophenyl)-5-nitrobenzamide 54253-05-3 C13H8Cl2N2O3 311.124 2-氯-5-硝基三氟甲苯 4-chloro-3-(trifluoromethyl)nitrobenzene 777-37-7 C7H3ClF3NO2 225.555 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氯-5-硝基苯甲酸甲酯 2-chloro-5-nitro-benzoic acid methyl ester 6307-82-0 C8H6ClNO4 215.593 2-氯-5-硝基苯甲酸乙酯 ethyl 2-chloro-5-nitrobenzoate 16588-17-3 C9H8ClNO4 229.62 —— N-(3-Carboxy-4-chlor-phenyl)-hydroxylamin 3133-03-7 C7H6ClNO3 187.583 2-氯-5-硝基苯甲酸叔丁酯 t-Butyl 2-chloro-5-nitrobenzoate 55233-05-1 C11H12ClNO4 257.674 —— n-Butyl 2-chloro-5-nitrobenzoate —— C11H12ClNO4 257.674 —— 2-Methylpropyl 2-chloro-5-nitrobenzoate 1256568-11-2 C11H12ClNO4 257.674 2-氯-5-硝基苄醇 2-chloro-5-nitrobenzyl alcohol 80866-80-4 C7H6ClNO3 187.583 —— hexyl 2-chloro-5-nitrobenzoate 1256568-09-8 C13H16ClNO4 285.727 —— heptyl 2-chloro-5-nitrobenzoate 1256568-07-6 C14H18ClNO4 299.754 —— octyl 2-chloro-5-nitrobenzoate 1256568-06-5 C15H20ClNO4 313.781 2-氯-3,5-二硝基苯甲酸 2-chloro-3,5-dinitrobenzoic acid 2497-91-8 C7H3ClN2O6 246.564 间硝基苯甲酸 3-nitrobenzoic acid 121-92-6 C7H5NO4 167.121 —— cycloheptyl 2-chloro-5-nitrobenzoate 1256568-08-7 C14H16ClNO4 297.738 5-氨基-2-氯苯甲酸 2-chloro-5-aminobenzoic acid 89-54-3 C7H6ClNO2 171.583 2-氯-5-硝基苯甲酰氯 2-chloro-5-nitrobenzoylchloride 25784-91-2 C7H3Cl2NO3 220.012 2-氯-5-硝基苯乙酮 2'-chloro-5'-nitroacetophenone 23082-50-0 C8H6ClNO3 199.594 2-氯-5-硝基苯甲酰胺 2-chloro-5-nitro-benzamide 16588-15-1 C7H5ClN2O3 200.581 5-氨基-2-氯苯甲酸甲酯 methyl 2-chloro-5-aminobenzoate 42122-75-8 C8H8ClNO2 185.61 —— ethyl 2-(2-chloro-5-nitro-benzoyloxy)-2-methyl-propionate 134605-96-2 C13H14ClNO6 315.71 2-氯-N-甲基-5-硝基苯甲酰胺 2-chloro-5-nitro-N-methylbenzamide 85469-93-8 C8H7ClN2O3 214.608 2-氨基-5-硝基苯甲酸 2-amino-5-nitro-benzoic acid 616-79-5 C7H6N2O4 182.136 —— 2-chloro-5-nitrobenzohydrazide 134432-61-4 C7H6ClN3O3 215.596 5-氨基-2-氯苯甲酸乙酯 5-amino-2-chloro-benzoic acid ethyl ester 64401-55-4 C9H10ClNO2 199.637 —— 4,4'-dichloro-3,3'-azobenzenedicarboxylic acid —— C14H8Cl2N2O4 339.135 —— N,N-dimethyl-2-chloro-4-nitrobenzamide 60587-79-3 C9H9ClN2O3 228.635 —— 1-(9,10-dimethoxyanthracene-2-ylmethoxy)-11-(2'-chloro-5'-nitrobenzoyloxy)-3,6,9-trioxaundecane —— C32H34ClNO10 628.076 2-氯-5-硝基二苯甲酮 2-chloro-5-nitrobenzophenone 34052-37-4 C13H8ClNO3 261.664 3-[(2-氯-5-硝基苯基)甲氧基]吡啶 3-[(2-chloro-5-nitrophenyl)methoxy]pyridine 642084-74-0 C12H9ClN2O3 264.668 5-硝基水杨酸 5-Nitrosalicylic acid 96-97-9 C7H5NO5 183.12 —— N-ethyl-2-chloro-5-nitrobenzamide 117054-83-8 C9H9ClN2O3 228.635 N-苄基-2-氯-5-硝基苯甲酰胺 N-benzyl-2-chloro-5-nitrobenzamide 83909-69-7 C14H11ClN2O3 290.706 5-(乙酰基氨基)-2-氯苯甲酸 5-acetylamino-2-chloro-benzoic acid 719282-11-8 C9H8ClNO3 213.62 2-氯-5-硝基-4'-氯二苯甲酮 2-chloro-5-nitro-4'-chlorobenzophenone 70132-91-1 C13H7Cl2NO3 296.109 (2-氯-5-硝基苯基)(4-甲基苯基)甲酮 2-Chloro-5-nitro-4'-methylbenzophenone 35485-71-3 C14H10ClNO3 275.691 2-氯-N-异丙基-5-硝基苯甲酰胺 2-chloro-N-isopropyl-5-nitrobenzamide 85469-94-9 C10H11ClN2O3 242.662 2-溴甲基-1-氯-4-硝基苯 2-(bromomethyl)-1-chloro-4-nitrobenzene 52427-01-7 C7H5BrClNO2 250.479 —— 2-chloro-5-nitro-N-(n-propyl)benzamide 130674-22-5 C10H11ClN2O3 242.662 2-氯-5-硝基苯甲腈 4-chloro-3-cyanonitrobenzene 16588-02-6 C7H3ClN2O2 182.566 —— 2-(4-chlorophenylamino)-5-nitrobenzoic acid 61767-78-0 C13H9ClN2O4 292.678 2-肼基-5-硝基苯甲酸 2-hydrazineyl-5-nitrobenzoic acid 185556-56-3 C7H7N3O4 197.15 N-丁基-2-氯-5-硝基苯甲酰胺 2-chloro-5-nitro-N-butylbenzamide 68505-92-0 C11H13ClN2O3 256.689 —— 1,2-bis-(2-chloro-5-nitro-benzoylamino)-ethane —— C16H12Cl2N4O6 427.2 —— 2,2',4'-trichloro-5-nitro-benzophenone 111855-93-7 C13H6Cl3NO3 330.555 2-(甲基氨基)-5-硝基苯甲酸 2-(methylamino)-5-nitrobenzoic acid 3484-33-1 C8H8N2O4 196.163 —— N-(2-chloro-5-nitrobenzoyl)thiourea —— C8H6ClN3O3S 259.673 —— N-<(N,N-dimethylamino)ethyl>-2-chloro-5-nitrobenzamide 142439-48-3 C11H14ClN3O3 271.703 —— (2-chloro-5-nitrophenyl)(4-fluorophenyl)methanone 197653-87-5 C13H7ClFNO3 279.655 (2-氯-5-硝基苯基)(4-甲氧苯基)甲酮 2-chloro-4'-methoxy-5-nitro-benzophenone 70132-87-5 C14H10ClNO4 291.691 —— 2-chloro-N-hexyl-5-nitrobenzamide —— C13H17ClN2O3 284.743 —— 2-chloro-5-nitro-N,N-diethylbenzamide 67272-98-4 C11H13ClN2O3 256.689 —— N,N-dibenzyl-2-chloro-5-nitrobenzamide —— C21H17ClN2O3 380.831 —— N(β-phenethyl)-2-chloro-5-nitrobenzamide 19007-50-2 C15H13ClN2O3 304.733 —— 2-chloro-5-nitro-4'-ethoxybenzophenone —— C15H12ClNO4 305.718 2-氟-5-硝基苯甲酸甲酯 methyl 2-fluoro-5-nitro-benzoate 2965-22-2 C8H6FNO4 199.138 —— Methanone, (2-chloro-5-nitrophenyl)(3,4-dichlorophenyl)- 113456-95-4 C13H6Cl3NO3 330.555 2-甲氧基-5-硝基苯甲酸 2-methoxy-5-nitrobenzoic acid 40751-89-1 C8H7NO5 197.147 —— 2-chloro-5-nitro-N′-phenylbenzohydrazide —— C13H10ClN3O3 291.694 3-硝基水杨酸 3-nitrosalicylic acid 85-38-1 C7H5NO5 183.12 2-氯-5-硝基-N-苯基苯酰胺 2-chloro-5-nitrobenzanilide 22978-25-2 C13H9ClN2O3 276.679 —— 2-chloro-N-cyanomethyl-N-methyl-5-nitrobenzamide 205309-72-4 C10H8ClN3O3 253.645 —— 2-chloro-N-methoxy-N-methyl-5-nitrobenzamide —— C9H9ClN2O4 244.634 2-(二甲基氨基)-5-硝基苯甲酸 2-(dimethylamino)-5-nitrobenzoic acid 4405-28-1 C9H10N2O4 210.189 —— 2-(3-chlorophenylamino)-5-nitrobenzoic acid 90656-49-8 C13H9ClN2O4 292.678 —— 2-chloro-N-(4-chlorophenyl)-5-nitrobenzamide 54253-05-3 C13H8Cl2N2O3 311.124 —— benzyl 2-(2-chloro-5-nitrobenzamido)acetate 1061735-40-7 C16H13ClN2O5 348.743 2-(4-氨基苯胺基)-5-硝基苯甲酸 2-((4-aminophenyl)amino)-5-nitrobenzoic acid 63594-71-8 C13H11N3O4 273.248 5-硝基-N-苯基邻氨基苯甲酸 5-nitro-2-(phenylamino)benzoic acid 16927-50-7 C13H10N2O4 258.233 N-(4-硝基苯基)-5-硝基邻氨基苯甲酸 N-(4-nitrophenyl)-5-nitroanthranilic acid 730978-51-5 C13H9N3O6 303.231 —— N-[(N,N-dimethylamino)ethyl]-2-chloro-5-nitrobenzamide —— C11H14ClN3O3 271.703 —— 2-chloro-5-nitro-N'-[(pyridin-4-yl)carbonyl]benzohydrazide 392290-27-6 C13H9ClN4O4 320.692 2-氯-N-(4-甲基苯基)-5-硝基苯甲酰胺 2-chloro-5-nitro-N-(p-tolyl)benzamide 292638-37-0 C14H11ClN2O3 290.706 —— 2-chloro-5-nitro-N-cyclohexylbenzamide 328259-17-2 C13H15ClN2O3 282.727 2-叠氮-5-硝基苯甲酸 2-azido-5-nitrobenzoic acid 54974-61-7 C7H4N4O4 208.133 —— 2-(2-chlorophenylamino)-5-nitrobenzoic acid —— C13H9ClN2O4 292.678 —— N-<2-Chlor-5-nitro-benzoyl>-<3>picolylamin 92044-68-3 C13H10ClN3O3 291.694 —— 2-chloro-N-(cyclohexylmethyl)-5-nitrobenzamide 852038-05-2 C14H17ClN2O3 296.754 —— 5-nitro-2-(2-tolylamino)benzoic acid 60645-16-1 C14H12N2O4 272.26 —— 2-((2-carboxyphenyl)amino)-5-nitrobenzoic acid 94654-69-0 C14H10N2O6 302.243 —— 5-nitro-2-(3-tolylamino)benzoic acid —— C14H12N2O4 272.26 —— 5-nitro-2-(4-tolylamino)benzoic acid 60645-17-2 C14H12N2O4 272.26 —— 5-nitro-2-(propylamino)benzoic acid —— C10H12N2O4 224.216 2-氯-5-硝基-N-4-吡啶基苯甲酰胺 T0070907 313516-66-4 C12H8ClN3O3 277.667 —— 2-(β-hydroxyethylamino)-5-nitrobenzoic acid 104144-90-3 C9H10N2O5 226.189 —— 2-(4-chlorophenoxy)-5-nitrobenzoic acid —— C13H8ClNO5 293.663 —— (2-chloro-5-nitrophenyl)(morpholino)methanone 142439-65-4 C11H11ClN2O4 270.672 —— N-(4-Ethylphenyl)-(2-chloro-5-nitrophenyl)carboxamide 346721-92-4 C15H13ClN2O3 304.733 2-氯-N-(4-羟基苯基)-5-硝基苯甲酰胺 N-(4-Hydroxyphenyl)-(2-chloro-5-nitrophenyl)carboxamide 22978-55-8 C13H9ClN2O4 292.678 —— N,N-dibutyl-2-chloro-5-nitrobenzamide —— C15H21ClN2O3 312.796 —— 2-(butylamino)-5-nitrobenzoic acid 83909-52-8 C11H14N2O4 238.243 —— 2-ethoxy-5-nitrobenzoic acid 57148-23-9 C9H9NO5 211.174 —— 2-(2-Carboxy-5-chloroanilino)-5-nitrobenzoic acid 197304-54-4 C14H9ClN2O6 336.688 —— N,N'-bis-(2-carboxy-4-nitrophenyl)-1,2-diaminoethane —— C16H14N4O8 390.309 —— 2-chloro-N-(4-fluorophenyl)-5-nitrobenzamide —— C13H8ClFN2O3 294.67 —— (2-chloro-5-nitrophenyl)(piperidin-1-yl)methanone —— C12H13ClN2O3 268.7 (2-氯-5-硝基苯基)(2,4-二甲基苯基)甲酮 (2-Chloro-5-nitrophenyl)(2,4-dimethylphenyl)methanone 113456-94-3 C15H12ClNO3 289.718 2-[(4-氯苯基)硫代]-5-硝基苯甲酸 2-(4-chloro-phenylsulfanyl)-5-nitro-benzoic acid 66949-29-9 C13H8ClNO4S 309.73 —— methyl 3-(2-chloro-5-nitrophenyl)-2-propenoate —— C10H8ClNO4 241.631 —— 2-(n-hexylamino)-5-nitrobenzoic acid 112887-30-6 C13H18N2O4 266.297 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:描述:参考文献:名称:Iodo Derivatives of Diphenyl Ether. I. The Mono- and Certain Diiodo-Derivatives of Diphenyl Ether, and of 2- and 4-Carboxy Diphenyl Ethers1摘要:DOI:10.1021/ja01316a035
-
作为产物:描述:参考文献:名称:设计,合成和生物学评估作为拓扑异构酶I抑制剂的3-取代的茚并异喹啉衍生物摘要:设计并合成了一系列新的茚并异喹啉衍生物。在HepG2,A549和HCT-116细胞系中评估了这些新型化合物的体外抗增殖活性。化合物9a,9b,10a,10c,10e,18a和18b表现出对三种测试癌细胞系的有效抑制活性。还测试了19种化合物在50μM下对Top I的抑制作用。在该浓度下,几乎所有测试的化合物都显示出有效的Top I抑制活性。最有效的化合物9a和10a 与HCPT和TPT相比,它具有更高的细胞毒性,并且在我们的生物学分析中,对Top I的抑制活性可与CPT相媲美。DOI:10.1016/j.bmcl.2015.12.014
文献信息
-
Npy antagonists, preparation and uses申请人:Botez Iuliana公开号:US20090233910A1公开(公告)日:2009-09-17The present invention concerns novel compounds, their preparation and their uses, therapeutic uses in particular. More specifically it concerns derivative compounds having at least two aromatic cycles, their preparation and their uses, in particular in the area of human or animal health. These compounds have an affinity for the biological receptors of neuropeptide Y, NPY, present in the central and peripheral nervous systems. The compounds of the invention are preferably NPY antagonists, and more particularly antagonists of sub-type NPY Y1, and can therefore be used for the therapeutic or prophylactic treatment of any disorder involving NPY. The present invention also concerns pharmaceutical compositions containing said compounds, their preparation and their uses, as well as treatment methods using said compounds.本发明涉及新颖化合物,它们的制备和用途,特别是在治疗方面的用途。更具体地说,它涉及至少具有两个芳香环的衍生化合物,它们的制备和用途,特别是在人类或动物健康领域。这些化合物对存在于中枢和外周神经系统中的神经肽Y(NPY)的生物受体具有亲和力。本发明的化合物优选为NPY拮抗剂,更具体地说是NPY Y1亚型的拮抗剂,因此可用于治疗或预防涉及NPY的任何疾病。本发明还涉及含有所述化合物的药物组合物,其制备和用途,以及使用所述化合物的治疗方法。
-
3-Alkoxy-thianapthene-2-carboxamides申请人:Societe d'Etudes Scientifiques et Industrielles de l'Ile-de-France公开号:US03954748A1公开(公告)日:1976-05-04The 3-alkoxy-thianaphthene-2-carboxamides of this invention are effective for the treatment of mammals afflicted with emesis. When administered to dogs in dosages of 250 .mu.g/kg, compounds of this invention give 100% protection against vomiting normally induced by subcutaneous administration of apomorphine. The compounds of this invention also favorably modify behavior disturbances in mammals.这项发明的3-烷氧基噻吩并2-羧酰胺对患有呕吐症的哺乳动物有效。当以250微克/千克的剂量给狗服用时,这项发明的化合物可使狗免受皮下注射阿波莫啡引起的呕吐,保护效果达到100%。这项发明的化合物还有助于改善哺乳动物的行为紊乱。
-
Thioimides: New Reagents for Effective Synthesis of Thiolesters from Carboxylic Acids作者:Adam Henke、Jiri SroglDOI:10.1021/jo801319x日期:2008.10.3carboxylic acids are used as the precursors for the convenient synthesis of thiolesters in the phosphine mediated process. Cyclic and acyclic thioimides show equal efficiency, furnishing the desired thiolesters in good to excellent yields. The general, highly efficient transformation tolerates various functional groups and the resulting thiolesters are obtained in high purity after a simple separation. The
-
Inhibitory growth evaluation and apoptosis induction in MCF-7 cancer cells by new 5-aryl-2-butylthio-1,3,4-oxadiazole derivatives作者:Rashmin Khanam、Kamal Ahmad、Iram I. Hejazi、Ibrar A. Siddique、Vikash Kumar、Abdul Roouf Bhat、Amir Azam、Fareeda AtharDOI:10.1007/s00280-017-3414-6日期:2017.11regulation of apoptotic signaling pathways may lead to cancer formation. Subsequently, the synthesis of effective chemotherapeutic agents that can induce apoptosis in tumor cell has emerged as a significant approach in cancer drug discovery. METHODS The goal of this work is to develop a potential antitumor agent exerting significant inhibitory effects on cancer cell and low cytotoxicity, for which we focused背景技术癌症已经成为全球健康问题之一,并且它是威胁生命的疾病,其特征在于细胞不受限制地生长。尽管化学疗法的管理取得了各种进步,但是由于高毒性,副作用和发展的耐药性,目前的抗癌药物如阿霉素,天冬酰胺酶,甲氨蝶呤,长春新碱的使用仍然受到限制。凋亡是关键的细胞过程,凋亡信号通路的调控不当可能导致癌症形成。随后,可以在肿瘤细胞中诱导凋亡的有效化学治疗剂的合成已成为癌症药物发现中的重要方法。方法这项工作的目的是开发一种潜在的抗肿瘤药物,该药物对癌细胞具有显着的抑制作用,且细胞毒性低,为此,我们将重点放在1,3,4-恶二唑的结构特征上,因为它是现代药物化学中的优先支架,并具有抑制可能与实现细胞永生和致癌作用有关的生长因子,酶和激酶的能力。结果体外MTT筛选试验显示化合物5-氨基苯基-2-丁基硫代-1,3,4-恶二唑(5e)对MCF-7癌细胞表现出最高的抑制作用,IC50值为10.05±1.08 µM,
-
Tricyclic benzazepine vasopressin antagonists申请人:American Cyanamid Company公开号:US05532235A1公开(公告)日:1996-07-02Tricyclic compound of the general Formula I: ##STR1## as defined herein which exhibit antagonist activity at V.sub.1 and/or V.sub.2 receptors and exhibit in vivo vasopressin antagonist activity, methods for using such compounds in treating diseases characterized by excess renal reabsorption of water, and process for preparing such compounds.
表征谱图
-
氢谱1HNMR
-
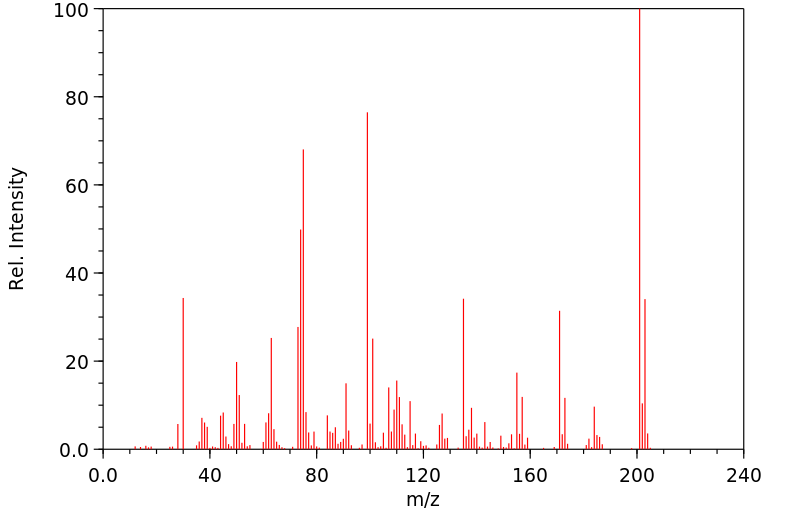
质谱MS
-
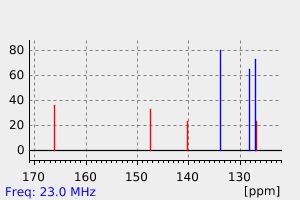
碳谱13CNMR
-
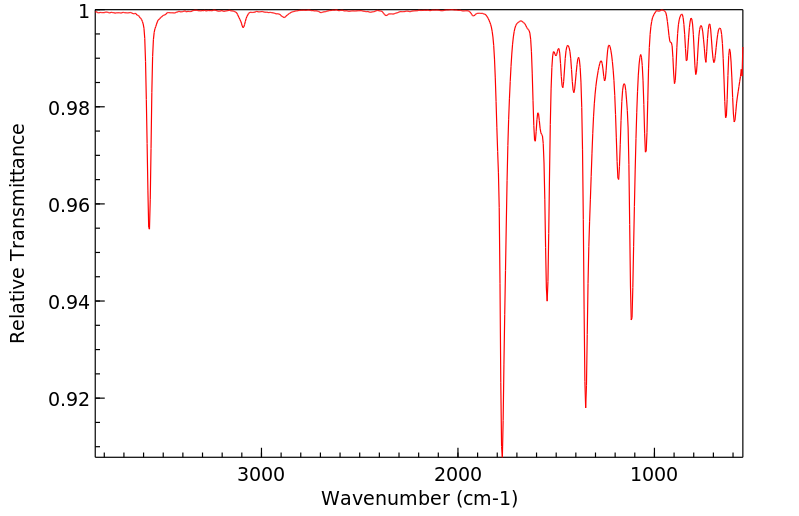
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫